One of the main pollutants produced by both automobile and industrial combustion emissions is Nitric oxide (NO). It has many adverse effects on the atmosphere, including formation of smog and loss of ozone. Despite a great amount of research, there are still many limitations in available methods which could eliminate this produced NO. One example is the removal of CO, unburnt hydrocarbons and NO utilizing three-way catalysts within automobiles. However, this has limitations, such as a restriction of a very precise efficient fuel/oxygen range. This problem has therefore impeded the advancement of leaner-burning engines with lower consumption of fuel.
Lots of powerplants add both oxygen and a reducing agent (e.g. ammonia), which stimulates catalytic reduction of NO:
| 4NH3 + 2NO + 2O2 → 6H2O + 3N2 |
(1) |
However, the perfect reaction, especially with automotive applications, is via the direct catalytic dissociation of NO:
However, no catalysts for this reaction have been constructed which have adequate activity at normal operating temperatures. The activity of a catalyst is measured in units of moles per gram of catalyst per second.
This article describes work which applies Raman spectroscopy to investigate the application of barium oxide upon magnesium oxide for the dissociation of NO. Within highly specific reaction conditions, exceptionally uncommon behavior has been noted within this system. Once barium is loaded at ≥11 mol%, the activity of the catalyst dramatically increases along with the temperature, preceding a sudden decline.
Additionally, this decrease in temperature is highly conditional upon the partial pressures of NO and extra O2. The reactivity of the catalyst starts to recover once higher temperatures over 150 °C are reached. However, the apparent difference in the temperature dependence suggests an alternate process is implicated in the two different temperature systems. Lots of research has been committed to comprehending this process in regards to surface phases and chemistry.
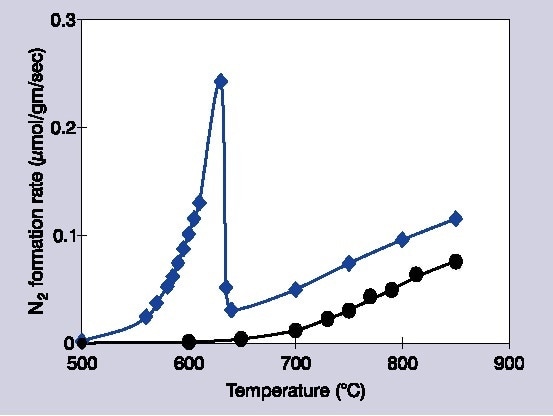
Figure 1. Decomposition rates of 1% NO/He over Ba/MgO catalysts: circles indicate 1 mol% Ba/MgO, diamonds indicate 14 mol% Ba/MgO.
Analysis Methods
Theoretically, FTIR and Raman could both be utilized to assess the numerous species of barium which could improve this mechanism. However, Raman was selected, as the specific region of interest is between the temperatures of 600 °C and 700 °C, and FTIR is not useful in temperatures over 500 °C as it produces disproportionate emission of blackbody from the samples.
Experimental
Raman analysis was done in situ, utilizing 532-nm incident radiation with a Kaiser Optical Systems, Inc., HoloProbeTM* analyzer. This machinery has very high sensitivity which is vital as the active species are very likely to have small surface tensions. Therefore, the low laser powers (a few mW) must be utilized to prevent too much sample heating of this temperature-sensitive reactive system. Small wavelengths are favored as the produced fluorescence is not within visible excitation, and the Raman signal intensity is proportionate (1/λ4). This application of smaller wavelengths also stops the production of blackbody emissions. A Mk II Probe head was utilized for the laser excitation and signal collection stages, at a working distance of 50 mm. This setup is highly sensitive, which enables the spectra to be captured using 2.5 mW of laser light upon the sample. Assessments with thermocouples showed that this generates an increase in local temperature (<5 °C). Every experiment used 50 mg of the catalyst which was suspended upon a fused-quartz frit. Gases (NO, O2, He) then flowed through this system, which was encircled within a low volume (10 ml) cell to allow quick re-establishment.
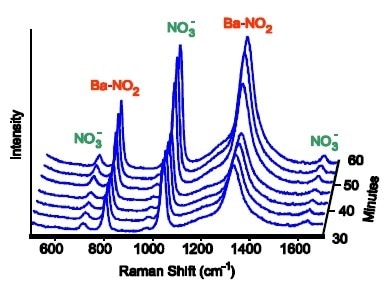
Figure 2. At 550 °C a barium-nitro species builds up, corresponding to an increase in catalytic activity.
Results and Discussion
Contingent upon the conditions of operating, the produced Raman spectra could be inspected for multiple species of “BaNO”. Such as; barium nitrate (Ba(NO3)2), barium nitrite (Ba(NO2) 2) and barium oxide with a nitro (NO2) ligand attached to the barium. There were parameters which varied, including the gas partial pressures and operating temperatures, to move to the low-activity regime from the high-activity regime. Each BaNO species had a different spectral intensity, however, the intensity of nitro species tightly correlated with the amount of catalyst activity in all the different conditions of operation. Therefore, the results suggest that the active intermediate which is accountable for the pack in activity is the barium nitro species.
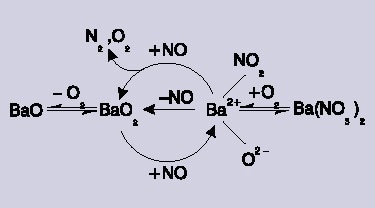
Figure 3. The catalytic reaction scheme. A barium-nitro reaction intermediate is formed by reaction of NO with barium peroxide (BaO2).
By assessing the Raman bands as a result of the nitrate and the crystalline barium peroxide, this allowed the understanding of the complex dependency on O2 pressure, allowing its inclusion into an final reaction scheme. Oxygen plays two different roles, firstly it produces barium peroxide leading to the production of the nitro species. Secondly, a large amount of O2 transforms the active nitro species to an inactive form of nitrate.
Further investigation of the catalyst surface morphology revealed that the active barium-nitro phase is not a single layer but acts like it is composed of numerous microcrystals. Current studies utilizing Raman spectroscopy are aiming the understand the chemistry behind the interaction between each of the phases.
Conclusion
The use of Raman spectroscopy in situ is a very invaluable tool which has allowed the understanding of a novel catalytic mechanism for NO removal. This variety of Ba/MgO catalyst, however, does not have an adequate catalytic activity for commercial applications, but this investigation has determined several qualities of catalytic intermediates which can lead to the decomposition of NO .
Reference:
- Xie, S.; Mestl, G.; Rosynek, M.P.; and Lunsford, J.H., “Decomposition of Nitric Oxide over Barium Oxide Supported on Magnesium Oxide. 1. Catalytic Results and in Situ Raman Spectroscopic Evidence for a Barium–Nitro Intermediate.” Journal of the American Chemical Society, Vol. 119, 1997, 10186.

This information has been sourced, reviewed and adapted from materials provided by Kaiser Optical Systems.
For more information on this source, please visit Kaiser Optical Systems.