One of the main components for the manufacture of silicone oil and rubbers is polyalkylsiloxanes, which are produced using methyl chlorosilanes. This methyl chloride is often commercially produced via a direct method, which creates a concoction of many compounds. The produced compounds include; basic methyl chlorosilane compounds, some Si–H– containing compounds (eHSiCl2, Me2HSiCl and HSiCl3), and simple chlorinated compounds (MeCl4 and SiCl4). However, it is the main product, dimethylchlorosilane, which is utilized in the manufacture of polydimethylsiloxanes.
The reaction is often optimized to produce the maximum amount of dimethylchlorosilane, even though the other by-products have several possible uses as raw materials. Distillation is utilized to remove the dimethylchlorosilane from the concoction of other products, as all the additional materials are liquids with a boiling range of 26–71 °C, but methyl chloride is a gas.
Throughout both the reaction and the distillation, the reaction products are monitored in order to guarantee the optimal creation of dimethylchlorosilane. This monitoring process is traditionally carried out via gas chromatography. This method, however, has numerous limitations. Firstly, it is highly time consuming (20 to 60 minutes per analysis) and secondly, the components need a high level of maintenance due to material corrosivity.
A possible alternate monitoring mechanism is Raman spectroscopy, which can provide feedback in five minutes and spectral differentiation of the main ingredients. It offers a large dynamic range, and enables high sensitivity, down to 100 ppm.
Experimental
A primary benefit of utilizing Raman spectroscopy for monitoring chemical reactions is that, through the use of fiber optics, the spectrometer can be set apart from the measurement site. This means that the spectrometer could be positioned within a non-classified area, such as a control room or a purged analyzer house.
For example, in one study a spectrometer was positioned within a basic NEMA-4 enclosure, which was rated for use in a NFPA Class 1, Division 2 environment. A HoloProbe™* fiber optic–based spectrometer was utilized, functioning with a frequency doubled 532-nm Nd:YAG laser. GC data was used to determine response factors for the selected Raman bands. The band intensities were normalized where the number of elements was previously known, allowing the calculation of additional components, whilst assuming a constant response.
Results
The produced chlorosilane spectra was adequately well differentiated in order to allow for measurements of each individual component and did not require multivariate modeling, despite a similar general appearance. A very strong Raman signal was produced, only requiring a few seconds to measure. Characterisation of the Si–H–containing compounds was managed due to the appearance of the hydride band, around 2200 cm–1.
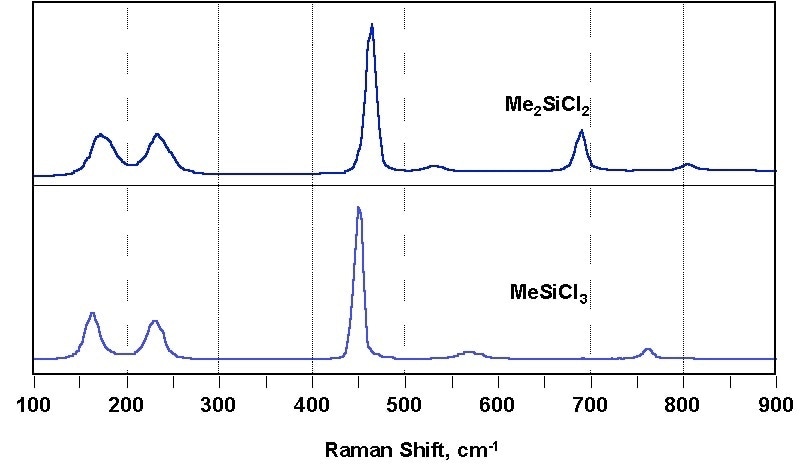
Figure 1. Example spectra of chlorosilanes
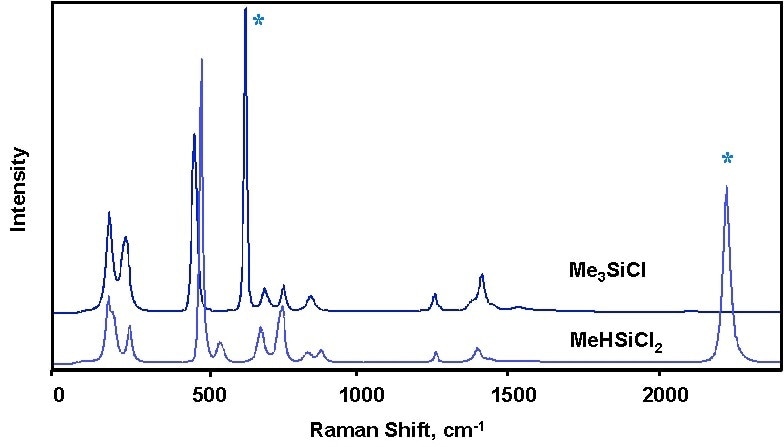
Figure 2. Comparison of chlorosilanes with and without Si-H.
The application of fiber optic probe coupling allows switching of locations during the process. A total of three streams were studied and showed a type of abnormal behavior; presented in the analyzed composition of both major and minor components. Data was sampled every five minutes, providing information on somewhat short-term variations happening in all of the selected streams. This commotion was caused by the instability in the distillation column as it started, or additional destabilizing events. Gas chromatography has an increased measurement time, however it does not give the temporal resolution which is needed to measure these alterations
A stream which contained two components (Me3SiCl and MeHSiCl2) was analyzed. During a period of seven hours, a cyclic change in relative composition was observed. This change happened during the recycle phase, in which the material contained within the overhead column and bottom streams was restored back into the original distillation column. This phenomenon is true, as shown by the identical copy of concentration profiles. The CG measurement, however, is too slow to identify these stages.
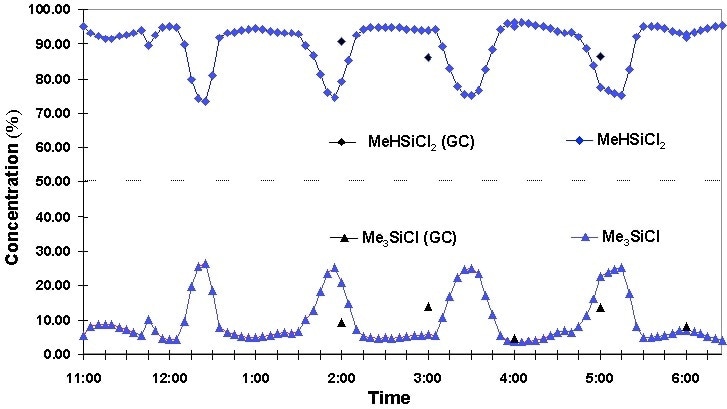
Figure 3. Process anomalies monitored by Raman.
Discussion
Numerous advantages of using Raman spectroscopy for reaction monitoring are outlined within this article, such as allowing remote monitoring of a chemically reactive and corrosive surrounding, fast measurement speeds, and the ability to measure numerous streams throughout the process. Within the measurement of multiple streams, multiplexing is made easy by the CCD configuration of the HoloProbe* instrument. This method allows the measurement of changes in numerous processes as they are happening, with excessive sensitivity between 100–1000-ppm.
Reference
- Lipp, E.D. and Grosse, R.L, “On-line Monitoring ofChlorosilane Streams by Raman Spectroscopy,” pplied Spectroscopy, Vol. 52, No. 1, January 1998.

This information has been sourced, reviewed and adapted from materials provided by Kaiser Optical Systems.
For more information on this source, please visit Kaiser Optical Systems.