Fluorine—a major element in industrial chemistry—is used in numerous applications such as polymers, agrochemicals, pharmaceuticals, solvents, and surfactants.
It is projected that fluorine is present in over 20% of all pharmaceutical compounds. This comprises some of the significant and commercially available drugs like Paxil (paroxetine) and Prozac (fluoxetine). Nuclear magnetic resonance (NMR) spectroscopy is a valuable method used for measuring compounds that contain fluorine, specifically organofluorine compounds. Moreover, after 1H and 13C NMR, 19F is the most common nucleus examined by this method. 19F nuclei are essentially spin ½ nuclei and known to have a high gyromagnetic ratio, that is, they have a high receptivity for NMR measurements.
With 100% natural abundance, the 19F isotope gives high NMR sensitivity. The resonance frequency of 19F on X-Pulse—a 1.45T magnet—is 56.76 MHz, which is adequately close to 1H resonance frequency such that the same probe can be used to measure the 1H and 19F spectra.

Measuring 19F spectra on X-Pulse
X-Pulse—a high-performance benchtop NMR spectrometer—does not need any external services like compressed air or liquid cryogens, and can be placed in the lab rather than in a dedicated NMR facility. Spectra can be acquired within a few minutes using regular 5-mm NMR tubes. Using the same probe, a range of spectra has been acquired to illustrate the capability of 19F and 1H, as well as the performance of the instrument.
Trifluorotoluene (TFT) serves as a valuable reference material for 19F NMR spectroscopy and can be utilized for 1H measurements in a manner similar to tetramethylsilane (TMS).
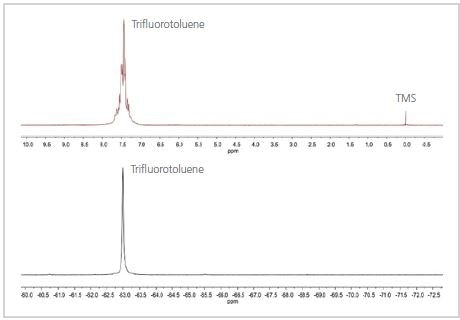
Figure 1. 1H (top) and 19F (bottom) spectra of trifluorotoluene
The 19F and 1H spectra of TFT are shown in Figure 1. As shown in Figure 1, the 19F spectrum contains a single peak because the three F nuclei are not very close to any of the H nuclei in the molecule, but are in a similar chemical environment. The 1H spectrum is more intricate because the H nuclei on the aromatic ring are not similar and the homonuclear couplings cause the aromatic peak to split.
As shown in Figure 2, the spectrum is a combination of two fluorine-comprising chemicals—trifluoroethanol and TFT, which is mostly utilized as a reference material for 19F spectra. TFT appears as a single robust peak with a chemical shift at –63.72 ppm with CFCl3 set at 0 ppm. Moreover, it is a single peak because the structure contains three similar F nuclei separated from any other nuclei that would adhere to it.
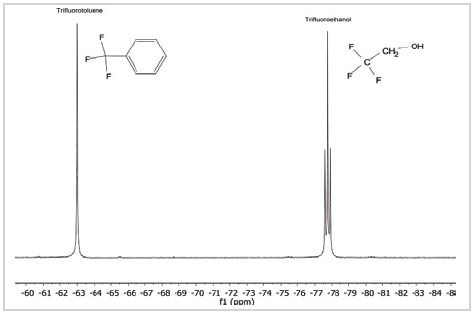
Figure 2. 19F spectrum of a mixture of trifluorotoluene and trifluoroethanol
By comparison, the peak in the spectrum caused by trifluoroethanol, at –77.7 ppm, is divided into three. This is because the 19F nuclei binds with the 1H nuclei on adjacent carbon atoms in the molecule, just as 1H nuclei would bind with other adjacent 1H nuclei in the molecule.
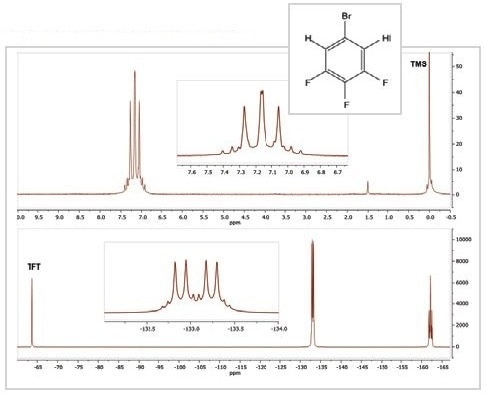
Figure 3. 1H (top) and 19F (bottom) spectra of 5-bromo-1,2,3-trifluorobenzene
The 19F and 1H spectra of the compound 5-Bromo-1,2,3-trifluorobenzene are shown in Figure 3. The 1H spectrum contains one multiplet of peaks arising from the two similar 1H nuclei and their couplings with 79Br, 19F, and 81Br nuclei inside the molecule.
Furthermore, the 19F spectrum contains a pair of resonances, each of which is a multiplet because of the two distinct chemical environments of the 19F nuclei. While the resonance at ~ –133 ppm results from the fluorine atoms in positions 1 and 3 on the molecule, the resonance at –162 ppm results from the fluorine in position 2.
All these resonances exhibit an intricate coupling pattern because of the presence of other nuclei on the aromatic ring. The triplet of triplet pattern at –162 ppm is the most easily recognized one; it emerges because the fluorine at position 2 is linked to two equivalent hydrogen atoms and two equivalent fluorine atoms. If fluorine couples with bromine, this would be too weak to be resolved in the spectrum.
Ultimately, the 19F spectra of two distinct positional isomers of bromotrifluorobenzene are compared. The 19F spectra of 1-bromo-2,4,5-trifluorobenzene and 5-bromo-1,2,3-trifluorobenzene are shown in Figure 4.
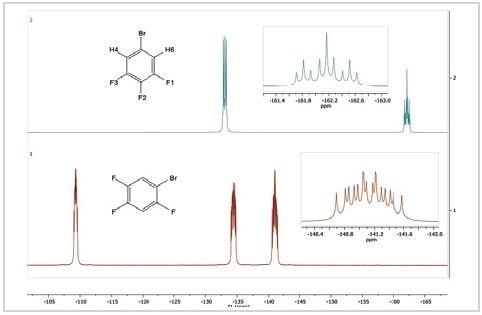
Figure 4. 19F spectra of 5-bromo-1,2,3-trifluorobenzene (top) and 1-bromo-2,4,5-trifluorobenzene
The spectrum of 1-bromo-2,4,5-trifluorobenzene displays three 19F resonances in contrast to the spectrum of 5-bromo-1,2,3-trifluorobenzene. This is because all fluorine atoms on the aromatic ring are in different chemical settings. They all display intricate coupling patterns because each contains two different hydrogen atoms and two different fluorine atoms and possibly a bromine atom as coupled neighbors.
Summary
The data provided in this article shows that benchtop NMR is a useful analytical tool for determining 19F spectra. The tool offers valuable data on fluorine chemistry. With the X-Pulse NMR spectrometer, both 1H and 19F spectra of a sample can be measured within a few minutes by making use of the same probe.

This information has been sourced, reviewed and adapted from materials provided by Oxford Instruments Magnetic Resonance.
For more information on this source, please visit Oxford Instruments Magnetic Resonance.