
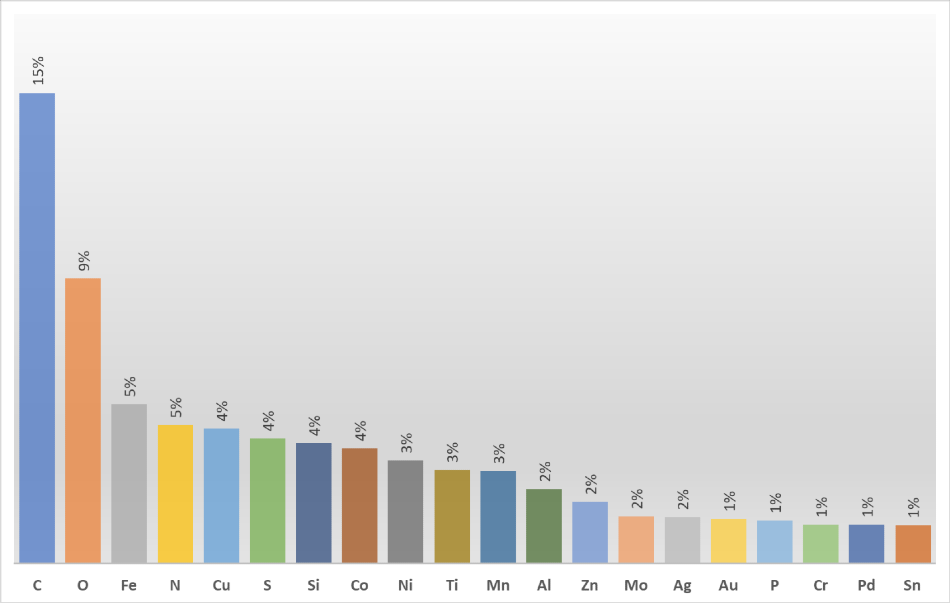
For over 50 years researchers all over the world have used X-ray photoelectron spectrometers to solve multiple problems. They naturally address different resources to gain better insight into the results of surface analysis experiments. One of these insights is the periodic table of XPS. It includes a wealth of XPS information, organized by element, which is extremely helpful once you try to understand why certain chemical elements are present on the surface being analyzed. By looking at the internet traffic, educated guesses can be made about what types of materials and, most importantly, what types of problems are being investigated by surface analysis professionals around the globe!
Carbon
Carbon is number one on the list as it is vital in so many applications, making it likely to be involved in a large number of experiments. The nature of X-ray photoelectron spectroscopy also gives a reason why carbon is seen so often.
XPS instruments are very surface sensitive and can detect the composition of the outermost atomic layers of a sample. Carbon compounds that are present in the air (for example, carbon dioxide and organic compounds from the inhabitants of the lab) are extremely likely to adhere to the surface of the majority of samples.
Most samples possess a layer of carbon on the surface. Generally, it appears as a mix of carbon-oxygen and carbon-carbon bonding in the C1s XPS spectrum, usually with a recognizable shape.
After the contamination is combatted, carbon is a compound that is utilized in a number of materials: from engineered polymers for lightweight transport to Li-ion batteries.
A lot of funding is being put into research into carbon nanomaterials, like graphene and carbon nanotubes, which are still in their early stages of application development. Market players in sports equipment and apparel are usually “early adopters” of those developments.
Keep in Mind: Care must be taken to remove the 2-3 nm of carbon contamination from a surface. Gentle ion bombardment from the system ion source (cluster ions being particularly good) will typically work, but it is a good idea to monitor the material below to make sure that no chemical changes are happening.
Oxygen Iron
Oxygen is second, but from a materials analysis perspective, it is always going to be present as it is bonded to something else. For example, number three on the list, which is iron. As one of the key components of steel, researchers are always trying to find the most reliable ways to protect steel surfaces from corrosion.
The outer oxide layer will comprise of a mixture of elements in different composition to the bulk, depending on the steel composition. Understanding the ratio of the different oxides at the surface helps to understand why a surface is resistant to corrosion – a property which is vital when choosing the right material for a pipeline, whether it is for gas, oil, or water.
Keep in Mind: As around half of last weeks' top ten are metals, it is probable that they are at least in part being investigated in combination with oxygen. Bear in mind that as oxidation states are crucial when analyzing metals, it is wise to pay attention to the additional information produced in a spectrum. Satellite features, like those seen for iron, or other effects like multiplet splitting, can be very diagnostic to help with identifying oxidation states or in some cases, even compounds.
Nitrogen
As is the case with oxygen, nitrogen will be present in a sample bonded to something else. For example, nitrides are extremely useful compounds, it is possible to see an element that has multiple uses when it is in a compound with nitrogen.
Silicon nitride is a tough, transparent material, as a component in semiconductor devices and commonly employed to supply scratch resistance. Architectural glass is one application of this material, where numerous ultra-thin layers of metals and oxides are applied to supply properties for temperature regulation (so-called low-e glass).
These layers require protection, which must be transparent, so a thin layer of silicon nitride may be applied. How the layer interacts with the layers below is also vital, and a method such as XPS depth profiling can be utilized to investigate if the layer structure used is stable.
Keep in Mind: Some nitrogen-containing materials like polyimide can be very sensitive to XPS surface analysis. To ensure reliable measurement of these samples, it is a good idea to gather data from multiple points and average together instead of gathering it at a single position.
What other applications might be key to the analysis of the elements shown? What other tips might people looking at compounds of these elements benefit from? Share your experience and your thoughts in the comments!

This information has been sourced, reviewed and adapted from materials provided by Thermo Fisher Scientific – X-Ray Photoelectron Spectroscopy (XPS).
For more information on this source, please visit Thermo Fisher Scientific – X-Ray Photoelectron Spectroscopy (XPS).