Unwanted reactions occur inside batteries that are used or stored, leading to degradation of performance. Surface coatings can be used to slow or passivate a number of these unwanted reactions, like lithium inventory loss, transition metal dissolution, and solid electrolyte interphase (SEI) growth.

Image Credit: Shutterstock/ Flegere
The Atomic Layer Deposition (ALD) process supplies the most precise, scalable, best performing, reproducible and cost-effective coating process to decrease unwanted reactions and increase the performance of batteries.
ALD can be used on a large scope of cathode and anode powders to give benefits such as slower impedance growth, lower gas generation, longer cycle life and higher voltage utilization.
A number of Fortune 500 companies are currently optimizing ALD coatings for their specific electrode powders and batteries with the utilization of high throughput ALD systems which are able to process 3,000 kg – 30,000 kg of powder each day (~10,000 tons per year per system) for <$1/kg total coating costs (CAPEX + OPEX).
ALD Enhances Battery Materials
ALD coatings on anode and cathode powders enhance battery performance and ALD coatings stabilize materials from the surface. The stabilizing nature of ALD coatings reduce SEI formation, decrease metal dissolution and lower lithium inventory loss.
Depending on the application, these effects give the following benefits:
- Longer calendar lifetime
- Higher voltage operation (and associated higher capacity)
- Lower impedance growth over cycling, resulting in higher capacity after cycling
- Less gas generation
- Enhanced safety (higher thermal runaway onset temperature, ARC measurement, etc.)
- Longer cycle life
These advantages have been seen in the following materials:
Anode Powders
Si/C composites, synthetic graphite, natural graphite, silicon
Cathode Powders
NCM (>80% Ni, 811, 721, 622, 532), LCO (at 4.5 V, 4.6 V), NCA (>80% Ni, >90% Ni), LMO (spinel and non-spinel types), LMNO
When using coatings on cathode powders, the higher the nickel (Ni) concentration, the less stable the cathode materials and the more crucial the surface coating. ALD coatings provide the most benefits against other coating techniques for high Ni materials.
Even a very thin (less than a nanometer) ALD coating can supply higher voltage performance and significantly enhanced cell cycle lifetimes for coatings on anode powders.
When combining a coated anode with a coated cathode, even more advantages can be seen for certain systems such as LCO/graphite systems. These include higher capacity retention over cycle life and higher initial specific discharge capacities.
Various ALD Chemistries are Possible
ALD coatings of numerous materials have been demonstrated to enhance battery performance in different ways and in different applications. The main chemistry of choice for most processes is aluminum oxide and it can enhance battery performance metrics outlined in this article.
Many companies are now exploring advanced coatings like those designed to have higher transport numbers and Li Ion mobilities. These advanced ALD coatings are usually applicable to hybrid batteries containing solid state components, lithium ion batteries and fully solid-state battery systems.
Advanced ALD coatings under current exploration for battery materials include metal phosphates, metal fluorides, metal oxides (other than aluminum oxide) and lithium metal oxides. Advanced ALD coatings, which can also be considered for battery materials include hybrid oxide/carbon coatings, polymer coatings and sulfur-coating coatings, to name a few.
Example Data of ALD-Coated Battery Materials
Recent coatings have shown particular benefit for fast charging of NMC 811 and high voltage operation of LCO, although ALD coated materials exhibit benefits on multiple battery materials. This recent work, which can be observed below, is crucial for consumer electronics (LCO) and automotive batteries (NMC811).
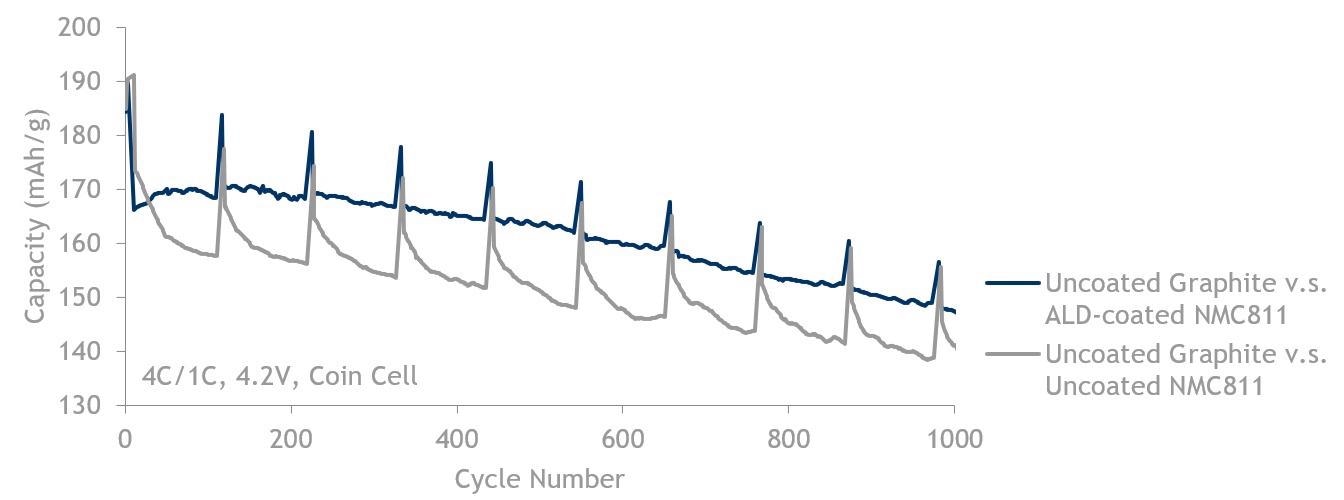
811 fast charge (4C/1C) data showing ALD-coated NMC811 (blue, top line) is better than NMC811 without ALD (grey, bottom line). Both are cycled against uncoated graphite up to 4.2 V.
Image Credit: Forge Nano
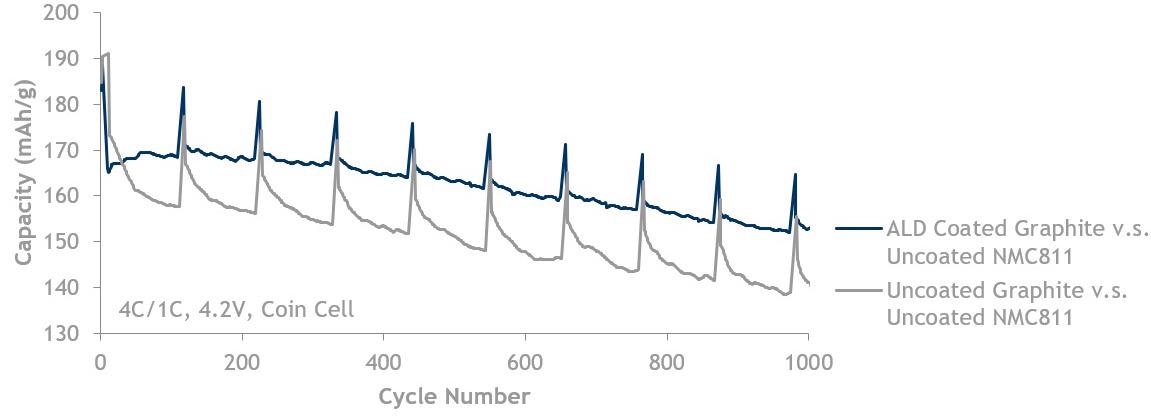
Graphite fast charge (4C/1C) data showing ALD coated graphite (blue, top line) is better than uncoated graphite (grey, bottom line). Both are cycled against uncoated NMC811 up to 4.2 V.
Image Credit: Forge Nano
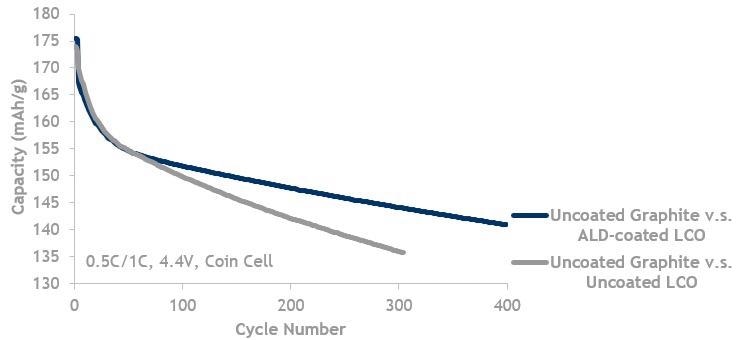
LCO lifecycle data showing ALD coated LCO (blue, top line) is better than LCO without ALD (grey, bottom line). Both are cycled against uncoated graphite.
Image Credit: Forge Nano
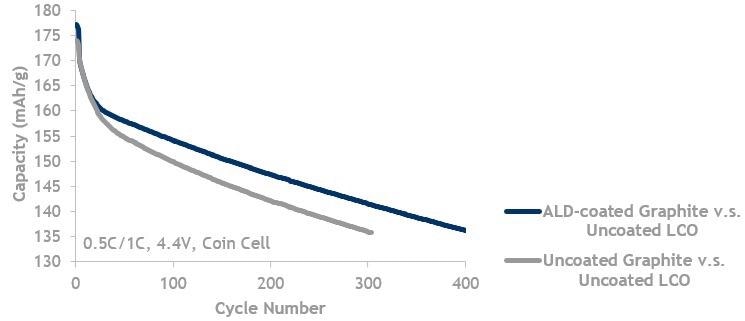
Graphite lifecycle data showing ALD coated graphite (blue, top line) is better than uncoated graphite (grey, bottom line). Both are cycled against uncoated LCO up to 4.4 V.
Image Credit: Forge Nano
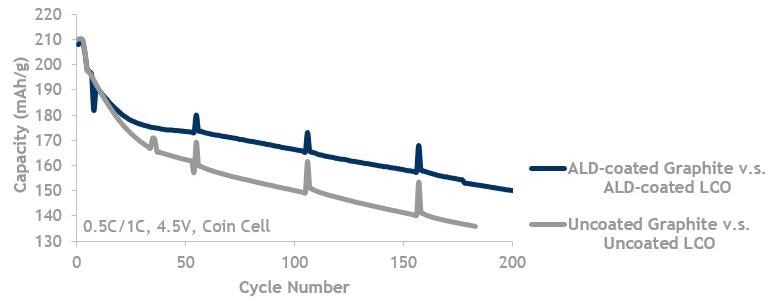
LCO and Graphite, high V (4.5 V) lifecycle data showing ALD coated graphite against ALD-coated LCO (blue, top line) is better than uncoated graphite (grey, bottom line) cycled against uncoated LCO.
Image Credit: Forge Nano
40 Ah Automotive Pouch Cells (NMC 811)
For screening materials, coin cells are a great method, but larger cells are needed to understand the value ALD coatings can bring to battery materials fully. 40 Ah automotive pouch cells have been produced with ALD coatings and electrochemical testing is currently being performed. The aim of this project was to demonstrate that ALD coatings could enhance larger cell’s safety and performance.
About Forge Nano
Forge Nano Inc. is the world leader in ultra-thin, surface modification technology for markets including catalysis, additive manufacturing, energy storage, electronic materials, adsorbents and is located just outside of Denver, Colorado and others. Forge Nano works together with strategic partners to jointly develop and integrate ALD-modified materials into commercial supply chains.
Forge Nano’s Capabilities
Over 15 in-house ALD systems for the coating of powders and objects, including the pilot, research, and commercial-scale systems, which can process anywhere from 1.0 g to 30,000 kg powder per day. Full coin cell (and soon, pouch cell) fabrication and testing lab, 250 Maccor and Arbin test channels, ~1/2 in environmental chambers
Ph.D. scientists, career scientists and certified Professional Engineers, the world’s most experienced and knowledgeable team for ALD onto powders, possess 20+ years’ experience designing and building powder ALD systems
Working with Forge Nano
Forge Nano assists customers daily with both R&D and commercialization of ALD enabled materials. For R&D, they provide research services for proofs of concept and sell their R&D equipment worldwide. For commercialization, they provide joint development of products, production equipment and IP licensing.

This information has been sourced, reviewed and adapted from materials provided by Forge Nano.
For more information on this source, please visit Forge Nano.