The content of hydrogen in a sample is determined by measuring its hydrogen content by weight. Be it before or after refining, the determination of this parameter is crucial in various oils because it is related to the combustion properties of the product.1
A sample bearing a larger hydrogen content can, in many cases, be used to achieve more efficient combustion.
The determination of hydrogen content in crude oils, fuels, and additives can be done through several existing methods. However, ASTM D5291 remains one of the most widely used.2
This method can be used for testing in a concentration range of 9-16 wt% for hydrogen; it should be noted that analyses outside of this range would require an alternative approach.
When analyzing volatile materials (e.g., gasoline), this specific method is not recommended. An elemental analyzer is required to use ASTM D5291, which means that compressed gases and routine maintenance and calibration are also necessary.
An elegant solution to determining the hydrogen content in oils has emerged in the form of nuclear magnetic resonance (NMR).
Information on the relevant constituents of these oils, such as aromatics,3 paraffins, and naphthenes, is afforded through the acquisition of a 1H spectrum.
The area under the curve of the many signals in a spectrum is related directly to how many atoms are in the sample, meaning that NMR is, inherently, a quantitative technique. Because of this, there has been a steady increase in the popularity of quantitative NMR (qNMR) as a powerful tool.4
The use of hexamethyldisiloxane (HMDSO), the internal calibrant used in this case, integrates the various regions and provides a quick and simple way of determining hydrogen content, by integrating regions of interest and making use of the following equation:
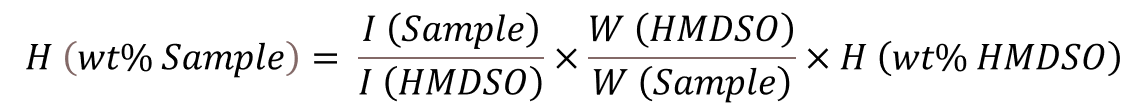 |
(1) |
Where,
I (Sample) is the sum of the sample’s integration areas,
I (HMDSO) is the integration area of HMDSO,
W (HMDSO) is the mass of HMDSO,
W (Sample) is the mass of the sample,
H (wt% HMDSO) is the hydrogen content of HMDSO.
It should be noted that it is of extreme importance for the masses of both HMDSO and the sample to be accurately recorded because these measurements have a significant impact on the results of these calculations.
Similarly, in order to obtain accurate integration results, it is crucial that the 1H NMR spectrum is properly processed.
Table 1. Comparison of running 3000 samples between elemental analysis (EA) (following ASTM D5291 for hydrogen content analysis in oils), and benchtop nuclear magnetic resonance (NMR) spectroscopy. Source: Nanalysis Corp.
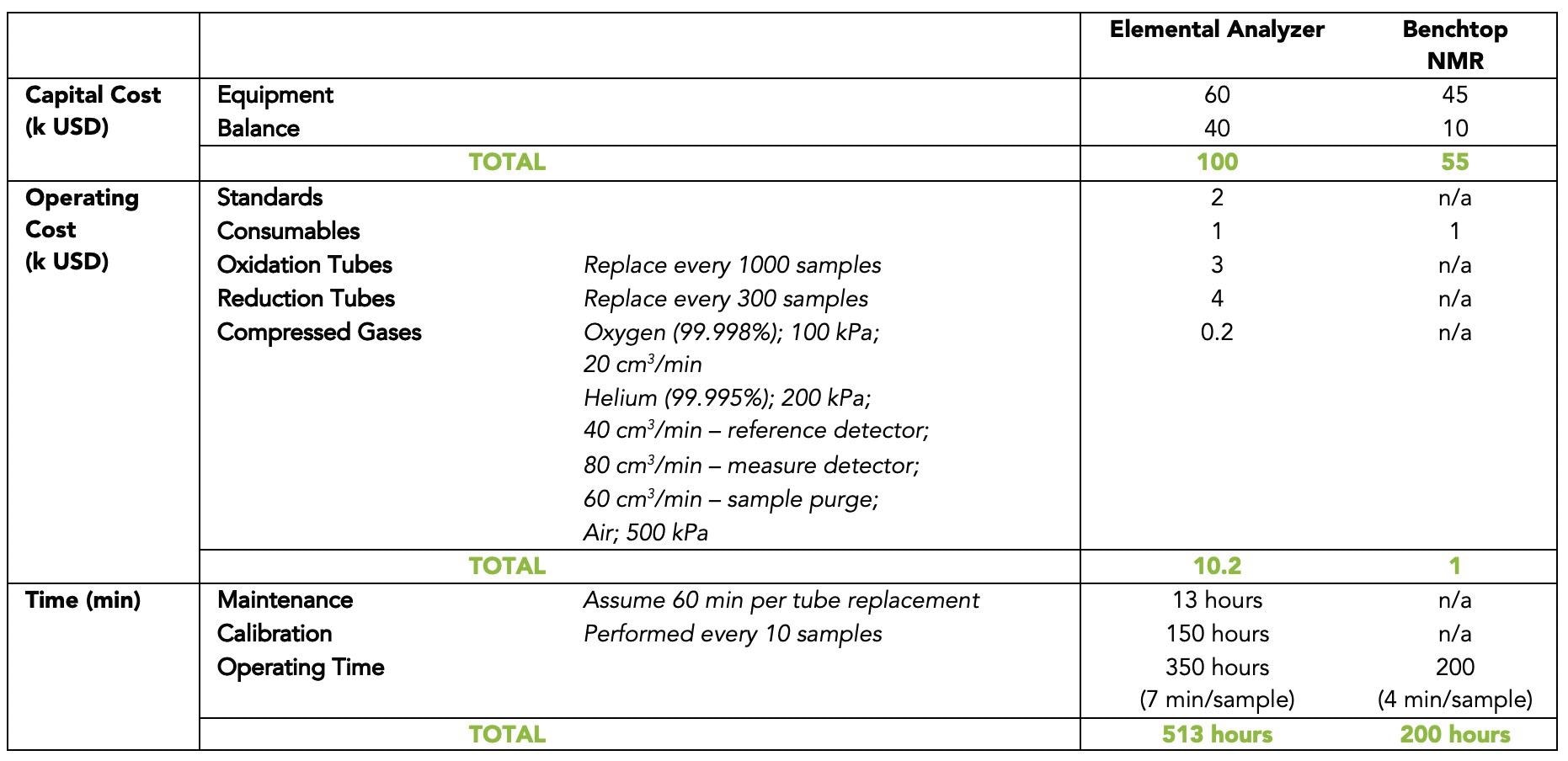
*Gas flow rates as described in ASTM D5291
The 1H NMR spectra for 1-octene and undecane were acquired in triplicate at 32 °C using a Nanalysis 60PRO instrument at 60.73 MHz proton frequency with the following parameters: spectral width, 40 ppm; spectral center, 10 ppm; a number of points, 16384; scans, 8; dummy scans, 0; interscan delay, 23 seconds; pulse angle, 90.00°.
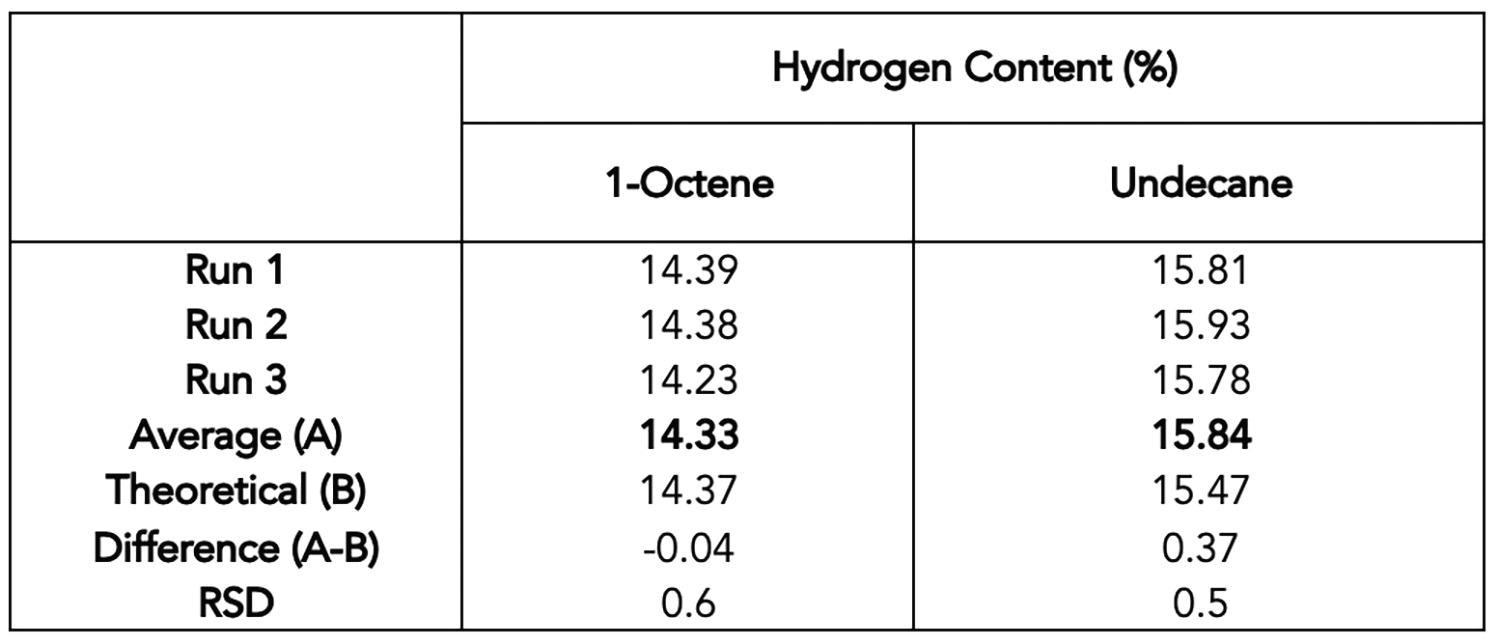
The theoretical values in 1-octene and undecane were compared to their hydrogen content as determined from Equation (1) and using the integration regions for I (Sample) that are highlighted in Figure 1. Table 2 summarizes the results.
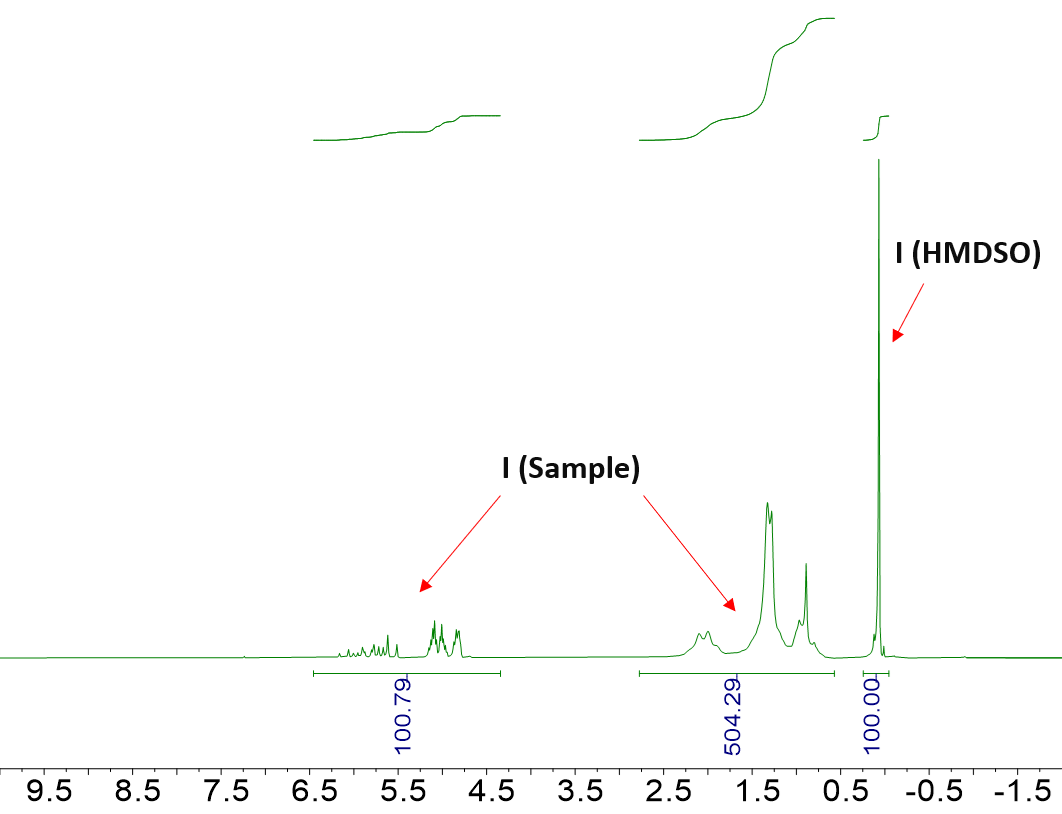
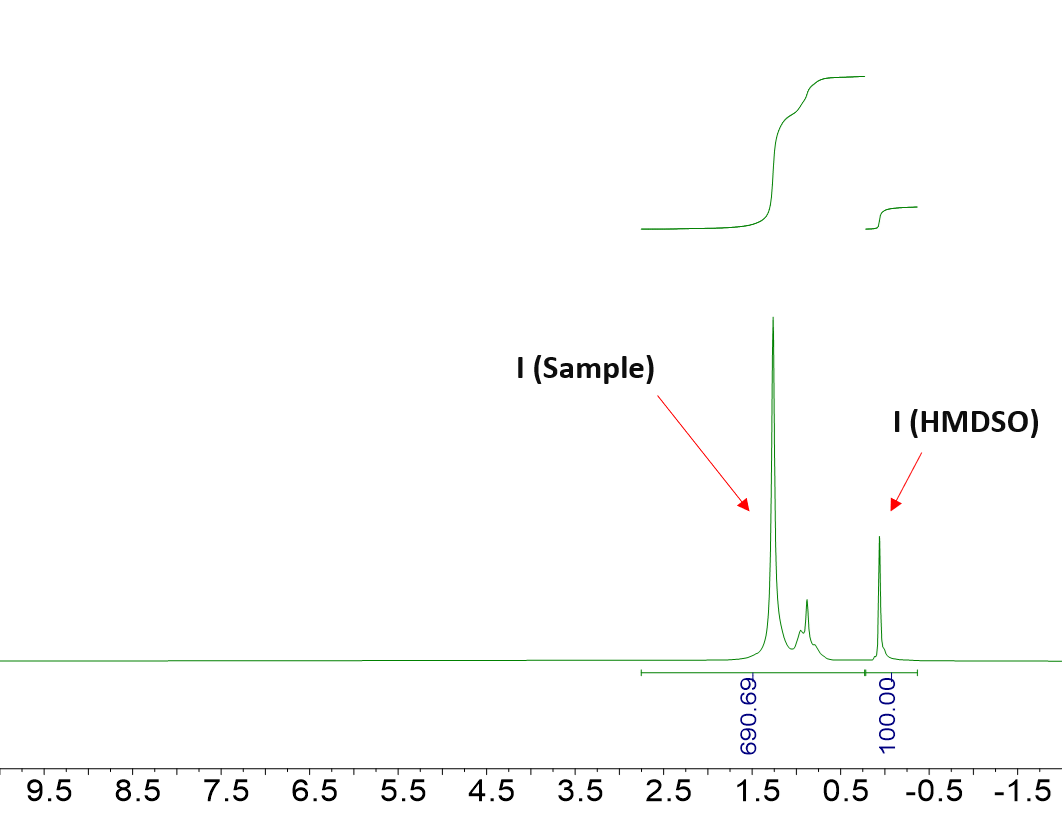
Figure 1. Representative 1H NMR spectrum of 1-octene (top) and undecane (bottom) in CDCl3. The integration regions used in the equation above, namely I (Sample) and I (HMDSO), are highlighted. Image Credit: Nanalysis Corp.
This method can be applied to a variety of crude oils, not just the undecane and 1-octene that were chosen for this study.
Table 2. Results of hydrogen content determination using the 60 MHz benchtop NMR for 1-octene and undecane. Source: Nanalysis Corp.
This approach can be expanded to include new types of samples once the T1 values are known and the integration region for I (Sample) has been determined.
qNMR is an incredibly useful technique to determine hydrogen content in various oils, as evidenced by these results.
Additionally, benchtop NMR minimizes the cost and waiting time of this work by allowing the analyses to be performed in-house, as opposed to outsourcing to a third party or sending these to another laboratory within the company.
References and Further Reading
- All, I.; Basit, M. A. Int. J. Hydrogen Energy 1993, 18, 1009-1011.
- Drews, A. W. Manual on Hydrocarbon Analysis (ASTM D5291): 6th Edition; American Society for Testing and Materials, 1998, 852-856.
- Mondal, S.; Kumar, R.; Bansal, V.; Patel, M. B. J. Anal. Sci. Technol. 2015, 6, 1-10.
- Bharti, S. K.; Roy, R. Trends Anal. Chem. 2012, 35, 5-26.

This information has been sourced, reviewed and adapted from materials provided by Nanalysis Corp.
For more information on this source, please visit Nanalysis Corp.