When discussing electrokinetic potential in colloidal dispersions, Zeta potential is the know scientific term. One component which affects the values of zeta potential is the chemical composition on the surface of a particle and the solution environment in which dispersion of the particles occurs.
The zeta potential and the intermolecular forces between the particles are closely related, which establishes the stability of a suspension system: a system with greater negative or positive zeta potential is typically more stable, while a suspension system with lower zeta potential close to 0 mv is less stable.
When the suspension system’s pH value fluctuates, the zeta potential may also change. In an acidic environment, the suspension system is typically positively charged and more negative charged in a more neutral environment.
This application note details the investigation of the relationship between the zeta potential and pH by measuring the zeta potentials of a commercially available, powdered coffee creamer in various pH environments. This creamer is a combination of polysaccharides, proteins and stabilizers.
Theory and Instrumentation
The technology used to measure the zeta potential is known as electrophoretic light scattering (ELS). In an ELS experiment, the sample is irradiated by a laser beam, where the scattered light is identified at a forward angle of 12°.
The diluted sample solution or suspension is treated by the application of an electric field at both ends of the sample cell, provoking the electrophoretic movement of the charged particles in the sample.
Due to the Doppler effect, the scattered light intensity undergoes a shift in frequency in contrast to the incident light. PALS analysis converts the scattered light signals with a frequency shift into phase shift.
The velocity of electrophoretic movement per unit electric field by the phase plot, which is identified as the electrophoretic mobility µ, is acquired. Using Henry’s equation, one can connect the zeta potential and ζ electrophoretic mobility µ as follows:
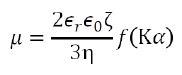
Where εr is the relative dielectric constant, ε0 is the solvent dielectric constant in vacuum, f(Κα) is the Henry function, Κ is the reciprocal Debye length, α is the particle radius, Κα refers to the ratio between the thickness of the double layer and the particle radius, and η is the solvent viscosity.
In this application note, BeNano 90 Zeta (Bettersize Instruments Ltd.) was utilized for the size and zeta potential measurement. The sample was illuminated using a laser beam with a wavelength of 633nm and a power of 10mW.
Avalanche photodiode detectors (APD) are assembled to accumulate scattered light signals at 90° for size measurement and 12° for zeta potential measurement, respectively.
Utilizing the phase analysis light scattering (PALS) method, the BeNano 90 Zeta was effective at establishing information relative to the zeta potential of samples with low electrophoretic mobility
Experiment
10mg of powdered coffee creamer was introduced to 100mL of distilled water and was dissipated for 15 minutes using a magnetic stirrer to achieve a stock solution. Then, the pH value of the stock solution was recorded at 8.3.
Subsequently, the stock solution divided equally into smaller portions across various sample cells, each with a volume of 3mL. The pH values of the solutions were modified by introducing each of the stock solutions to varying amounts of 0.025M HCl or 0.025M NaOH solution.
Table 1 details the varying volumes of HCl or NaOH solution as well as the relative pH values. The BeNano 90 Zeta’s built-in temperature control unit keeps the temperature of the samples stable at 25°C ± 0.1°C.
Table 1. Volumes of HCl or NaOH added and the corresponding pH values in each sample. Source: Bettersize Instruments Ltd
| Volume of HCl added [μL] |
Volume of NaOH added [μL] |
pH |
| 0 |
0 |
8.3 |
| 60 |
0 |
3.2 |
| 30 |
0 |
3.7 |
| 20 |
0 |
4.1 |
| 10 |
0 |
4.8 |
| 5 |
0 |
5.6 |
| 0 |
20 |
9.8 |
| 0 |
100 |
11 |
The zeta potential measurements were recorded using the folded capillary cell. Each sample was measured at least three times to make sure the results demonstrated repeatability.
Results and Discussion
The powdered coffee creamer’s zeta potentials were measured under different pH environments, the results of which are displayed in Figure 1 below.
As illustrated in Figure 1, the zeta potential of powdered coffee creamer solution demonstrates positive value in low pH ranges, suggesting that the surface of the particles transmitted positive charges in this pH range.
As pH value increased, there was a decrease in the zeta potential value, progressively approaching 0 mV when the pH was 5. After hitting the isoelectric point (IEP), the pH of the system continually increased as more and more NaOH was introduced, and the particles in the system began to carry negative charges.
In the higher pH region, the total values of zeta potential progressively increased in accordance with an increase in the pH value.
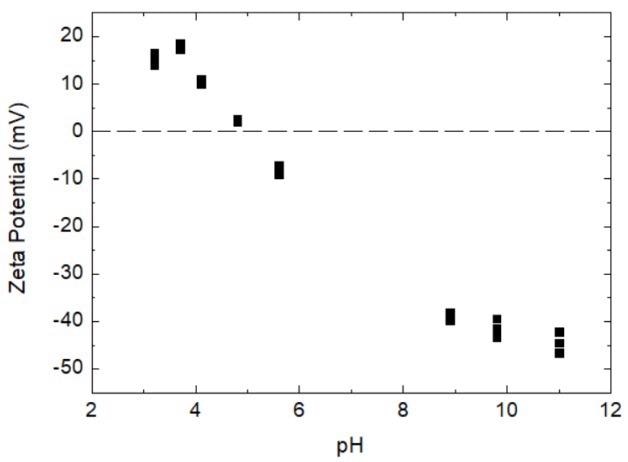
Figure 1. Scatter plot of powdered coffee creamer solution’s pH vs zeta potential. Image Credit: Bettersize Instruments Ltd
Conclusion
Employing PALS technique, the BeNano 90 Zeta has the capacity to detect signals even with low electrophoretic mobility with good efficiency and precision. The zeta potential results achieved using the BeNano 90 Zeta demonstrate excellent repeatability and accuracy even at low zeta potential, particularly in the ±10 mV range.

This information has been sourced, reviewed and adapted from materials provided by Bettersize Instruments Ltd..
For more information on this source, please visit Bettersize Instruments Ltd.
