The presence of flavors and fragrances is often subtle yet commonplace in our everyday lives. Like any other producers, the companies that manufacture flavors and scents are faced with numerous challenges to stay profitable while ensuring consistency and quality remain top priorities.
This is particularly true in the case of designing manufacturing facilities to develop and blend several products with commonly used equipment and process vessels. Introducing raw materials to process vessels that may harbor trace residues from the previous production batch could lead to:
- A change in the characteristics of the final product
- Jeopardizing product safety
Batches that have been compromised will be rejected or recalled, which in turn negatively impacts productivity and leads to profit loss. Therefore, it is crucial that the cleaning of the equipment between production batches is carefully undertaken to avoid cross-contamination, which could affect product safety and quality.
Consequently, it is absolutely crucial to validate equipment cleanliness. Some manufacturers monitor the pH or conductivity of the rinse water throughout the final stages of the equipment’s Clean-in-Place (CIP) cycle.
While this method may help reveal the presence of inorganic impurities, some contaminants will remain undetected. It is also typical practice for these manufacturers to use test methods that are subjective and approximate in nature, including visual inspections or surface swabs.
Challenge
A manufacturer of tobacco flavors in Asia conducted a validation of its cleaning process by manually swabbing wetted surfaces of its process vessels to run tests for traces of adenosine triphosphate (ATP).
ATP is a chemical compound that is present in all living cells, and the test is frequently used to detect microbial contamination. However, ATP may also be found in non-viable cells, thus showing inconsistency in the reliability and repeatability of the results.
The ATP values measured did not match the actual amount of residue present in the equipment. The flavor compounds produced at this facility do not support microbial growth, and therefore a negative ATP result does not necessarily relate to absent product residues.
Additionally, when swabbing the wetted surfaces of the equipment, there is a risk of reintroducing external contaminants.
To tackle the deficiencies of the ATP test method, the manufacturer was dependent on operator inspection, looking for product residues using their sense of smell.
Unfortunately, olfactory sensitivity is subjective, and the inspection outcome was not quantifiable. This makes it difficult for the manufacturer to exhibit control over the quality of the process, especially during customer audits.
Solution
Looking for a monitoring solution for cleaning verification, this manufacturer made the decision to investigate the benefits of TOC analysis. Monitoring for TOC facilitates rapid and accurate detection of flavor and fragrance residues, as these products are organic or possess organic components.
TOC analysis also catches trace amounts of hydrocarbon-based cleaning agents that may be left behind after CIP cycle completion, thus providing an overall snapshot of cleaning effectiveness.
The manufacturer selected the Sievers* M9 Portable TOC Analyzer from Veolia, recommended by a sister facility for its evaluation qualities. By relying on both UV and a powerful oxidizing reagent, the Sievers M9 TOC Analyzer can easily accomplish complete sample oxidation.
It also includes a unique membrane conductometric detection technology, which allows the analyzer to deliver consistent, accurate and precise readings in just two minutes, even when TOC levels are as low as sub-ppb concentrations.
The Veolia team assisted in the development of a TOC monitoring program for this facility by recommending that samples be collected at the equipment drain point during the final rinse step of the CIP cycle. A comparison of these measurements with the initial TOC baseline of the incoming rinse water provides a solid understanding of cleanliness.
If TOC values are shown to be elevated when exiting the rinse phases, this indicates the presence of residual products or cleaning agents in the equipment. At this point, the manufacturer can either extend the final rinse step or repeat the CIP cycle until the appropriate levels of equipment cleanliness are achieved.
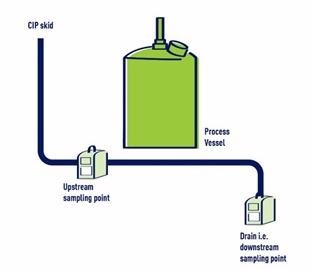
A depiction of the manufacturer’s TOC monitoring program. Image Credit: Veolia Water Technologies & Solution
*Trademark of Veolia; may be registered in one or more countries.
Results
The manufacturer determined that incoming rinse water values stabilized around one ppm following a period of data collection using the Sievers M9 TOC Analyzer. If the CIP cycle demonstrated its effectiveness, this value would be consistent post-rinse.
Should any product residues remain in the equipment, the TOC value would increase between 4 to 5 ppm, thus showing clear evidence of contamination. A review of the results demonstrated that TOC analysis offered:
- Accurate detection of product residue compared to conventional ATP testing
- A more dependable test method than olfactory inspections
- Quantifiable data
- Improved process understanding
Besides the added assurance of improved control over product quality, the manufacturer was also intrigued by the possibility of using TOC data to enhance existing CIP procedures, as this could generate cost-saving opportunities.
For instance, equipment used to produce an easy-to-clean product may only need a shorter rinse time or fewer cleaning chemicals.
A promising feature of the Sievers M9 Portable TOC Analyzer is its adaptability for use at-line or on-line monitoring. This feature gives the manufacturer flexibility when acquiring data in real-time - data that can facilitate key equipment release decisions.
This customer was also amazed by the simplicity of the installation of the Sievers M9 TOC Analyzer, particularly since it did not necessitate an external source of compressed air to function.
They discovered that the instrument was easy to maintain and only needed calibrating once a year. This facility has introduced TOC analysis as part of its quality control process.
In summary, TOC analysis with the Sievers M9 supplied this flavors manufacturer with a reliable, comprehensive and quantifiable method for verifying equipment cleanliness to protect the quality of its product.
TOC data is not only used to confirm the removal of trace product residues on vessel surfaces but also to verify adequate removal of cleaning agents and improve CIP cycles.

This information has been sourced, reviewed and adapted from materials provided by Veolia Water Technologies & Solutions.
For more information on this source, please visit Veolia Water Technologies & Solutions