Lithium-ion batteries (LIBs) have been extensively used in various applications due to their benefits, which include no memory effect, prolonged storage life, and low self-discharge rate. As a result of the need to store more energy, manufacturers have focused on the development of higher energy-density batteries in response to the rapidly increasing demand for LIBs in electrical products.

Image Credit: Bettersize Instruments Ltd.
The energy density of the anode can be increased substantially by optimizing the graphite particle size and shape. The ideal particle size for graphite is about 20 µm, which allows batteries to store more energy. Furthermore, the circularity of graphite has a direct impact on energy storage.
The higher the circularity of the graphite particles, the higher the tapped density. The optimal tapped density for graphite should be greater than 1 g/mL to hold more energy. The Bettersize laboratory used the Bettersizer S3 Plus to conduct an experiment to see how particle size and shape impact the energy density of LIBs.
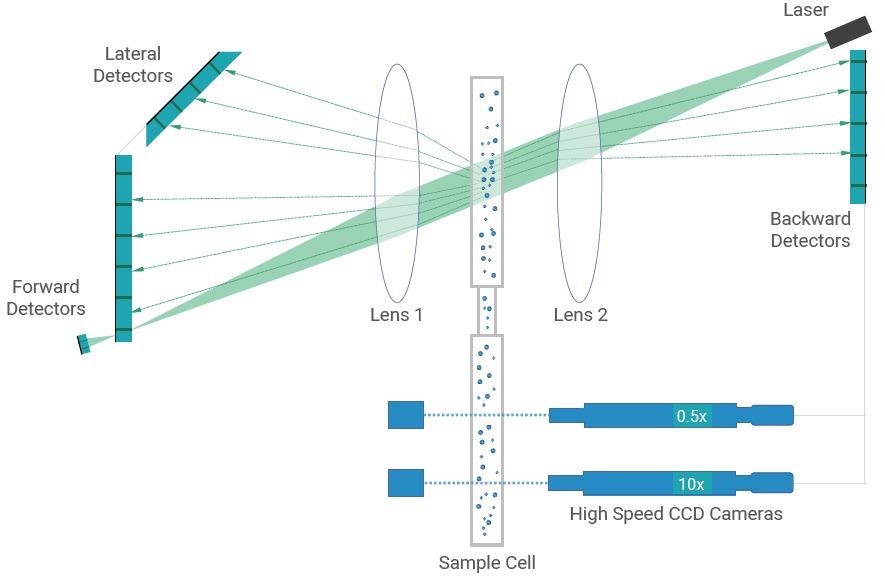
Figure 1. Optical System of the Bettersizer S3 Plus. Image Credit: Bettersize Instruments Ltd.
Results
Particle Size Distribution
The Bettersizer S3 Plus was used to quantify the particle size and particle size distribution of graphite samples using laser diffraction alone. Figure 2 depicts the particle size distribution of the three samples, while Table 1 lists the typical size values. The particle size gradually increases from Sample A to Sample C, as shown in Figure 2.
The three samples’ median size values (D50) are.6.804 μm, 15.98 μm, and 23.72 μm, respectively.
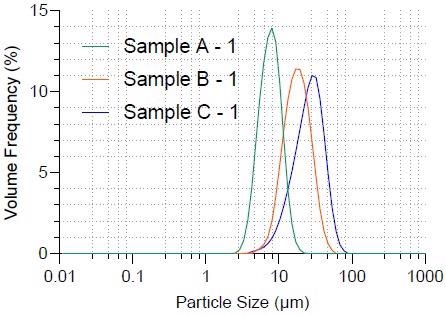
Figure 2. Particle size distribution of three graphite samples. Image Credit: Bettersize Instruments Ltd.
Table 1. Typical particle size values of graphite samples. Source: Bettersize Instruments Ltd.
| Sample |
D10 (μm) |
D50 (μm) |
D90 (μm) |
| Sample A |
4.264 |
6.804 |
10.49 |
| Sample B |
9.220 |
15.98 |
27.18 |
| Sample C |
11.60 |
23.72 |
39.98 |
Lithium-intercalation performance is affected by particle size, which is reflected in the LIBs’ initial reversible capacity, irreversible capacity, and cyclic performance. According to the findings, the initial irreversible capacity decreases as particle size increases.
The reversible capacity increases with particle size, peaking at 20 μm. Among the 13–80 μm graphite samples,1 the 20 μm graphite sample has the best energy-accumulation performance. D50 values in Sample B and Sample C are close to 20 μm, indicating that they will perform better in energy storage than in Sample A.
Particle Shape
Utilizing dynamic image analysis alone, the Bettersizer S3 Plus can evaluate shape parameters. Table 2 shows the results of measuring the circularity of three graphite samples.
Three graphite samples have medium circularity (C50) values of 0.862, 0.896, and 0.876, respectively. Sample B has a higher tapped density (1.01 g/mL) than the other two graphite samples.
Table 2. Circularity and tapped density of graphite samples. Source: Bettersize Instruments Ltd.
| Sample |
Circularity |
Tapped Density (g/mL) |
| C10 |
C50 |
C90 |
| Sample A |
0.813 |
0.862 |
0.917 |
0.85 |
| Sample B |
0.842 |
0.896 |
0.950 |
1.01 |
| Sample C |
0.774 |
0.876 |
0.924 |
0.95 |
When the anode has a high volumetric energy density, it tends to hold more energy, which is influenced by tapped density. An ideal tapped density for spherical graphite in anode manufacturing is greater than 1 g/mL.2
As particle size increases, so does the tapped density, as such Sample A has the smallest particle size and the smallest tapped density.
The tapped density can be affected not only by particle size but also by the shape of the raw materials. According to investigations, tapped density has a positive relationship with circularity,3 implying that Sample B (1.01 g/mL) has a higher tapped density than Sample C (0.95 g/mL).
The Sample B sample is expected to have the greatest energy-holding capacity of the three samples depending on the tapped density and particle size results.
Repeatability
Repeatability is a vital parameter of particle size measurement. Figure 3 shows that the particle size distributions of Sample C’s three replicates are almost identical.
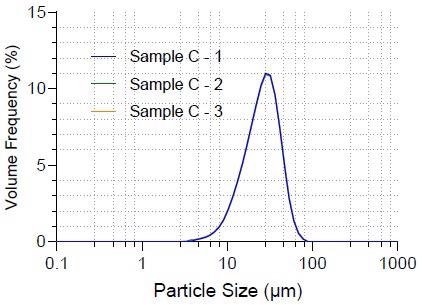
Figure 3. Repeatability of Sample C. Image Credit: Bettersize Instruments Ltd.
The repeatability of typical Sample C values is shown in Table 3. The D10, D50, and D90 size values have 0.28%, 0.08%, and 0.33% repeatability. As a result of its high repeatability, the Bettersizer S3 Plus is highly dependable.
Table 3. Repeatability of typical particle size values. Source: Bettersize Instruments Ltd.
| Samples |
D10 (μm) |
D50 (μm) |
D90 (μm) |
| Sample C-1 |
11.60 |
23.72 |
39.98 |
| Sample C-2 |
11.55 |
23.74 |
40.09 |
| Sample C-3 |
11.54 |
23.76 |
40.24 |
| Repeatability |
0.28% |
0.08% |
0.33% |
Conclusion
The energy storage capacity of the anode in LIBs is determined by particle size and shape, which should be managed and evaluated within an optimal range to enhance the efficiency of the manufacturing process.
The circularity and particle size of graphite should be measured using the dynamic image technique and the laser diffraction method, respectively, according to the Chinese Standard GB/T 38887-2020.4
To achieve the particle size and shape results separately, the conventional method needs two instruments. With laser diffraction and dynamic image technology combined in one instrument, the Bettersizer S3 Plus is the best choice for manufacturers looking for particle size and shape results in a single measurement.
References
- Chen, J., Zhou, H., Chang, W., & Ci, Y. (2003). Effect of Particle Size on Lithium Intercalation Performance of Graphite Anode. Acta Physico-Chimica Sinica, 19(03), 278- 282.
- Yan, C., Zhang, M., & Lin, Y. (2015). Effect of Graphite Particle Size on Tap Bulk Density. Non-Metallic Mines, 38(3).
- Teng, D., Li, P., Yuan, N., Lyu, J., Chen, J., Lin, L., & Chen, H. (2021). Process para meters optimization of natural graphite spheroidization. China Powder Science and Technology, 27(4).
- GB/T 38887-2020 - spherical graphite.

This information has been sourced, reviewed and adapted from materials provided by Bettersize Instruments Ltd.
For more information on this source, please visit Bettersize Instruments Ltd.
