This article explains how a process-relevant characterization of wet granules can facilitate the regulation of tablet Critical Quality Attributes (CQAs) and contribute to defining the Design Space in producing an over-the-counter (OTC) medicine.

Image Credit: Freeman Technology
Adopting a Quality by Design (QbD) approach for wet granulation requires manufacturers to comprehend the correlation between process variables, such as powder characteristics and equipment configurations, and the Critical Quality Attributes (CQA) inherent in the resultant product.
Manufacturers must establish a Design Space by grasping the impact of variations in the process on the granulate's properties and their subsequent influence on the ultimate product quality.
A robust Design Space also allows the control and manipulation of process variables to craft tablets with assured quality and targeted features.
Dynamic Characterization for QbD
Variations in material properties and process settings pose challenges and opportunities for manufacturers. Understanding how materials, which may exhibit batch-to-batch variations, behave within a manufacturing process characterized by potential fluctuations enables operators to devise a Design Space for operational guidance.
The capacity to transform challenges into opportunities in working with powders is significantly heightened when characterization techniques are employed, yielding repeatable and reliable results specifically relevant to the process conditions.
Unlike several established powder flow testing methods, such as tapped density, angle of repose, and shear cells, dynamic test approaches emulate typical process conditions, offering insights into material properties more likely to impact the ultimate product quality.
Evaluating Wet Granules
The dynamic testing method, employing a powder rheometer, proves effective in evaluating the characteristics of wet granules by gauging the resistance exerted by the granulate on a blade during its traversal through a sample.
This resistance is quantified as "flow energy," calculated from direct measurements of rotational torque and axial force as the blade moves. Various factors, including inter-particular friction, mechanical interlocking, the strength of capillary bonds, and cohesion between particles influence flow energy.
In high-shear wet granulation (HSWG), the incorporation of water and mechanical work (shear) yields larger, denser, and more adhesive granules, typically resulting in higher flow energy. The increased difficulty in displacing these larger, denser granules using the blade, coupled with their reduced compressibility, contributes to this higher flow energy.
Flow energy, often denoted as Basic Flowability Energy or BFE, thus becomes a function of the solid-to-water ratio, impeller speed, and binder temperature. This robust relationship between flow energy and process settings enables the identification of Critical Process Parameters and the determination of a Design Space.
Conducting tests in a wet state allows the application of this approach at an early stage in the manufacturing process. The following case study shows how a fully functional Design Space, based on dynamic flow properties, can be outlined for a wet granulation process, facilitating the targeting of Critical Quality Attributes (CQAs) in the final tablet.
Case Study: Defining Wet Granulation by Granule Quality
Two aspects of the Design Space definition were explored in the manufacturing of an over-the-counter (OTC) medicine.1
Firstly, the correlation between granulator variables and the resultant granule quality, assessed by rheological properties, was examined. Granules were produced using a pilot-scale High Shear Wet Granulation (HSWG) process, followed by drying in a fluidized bed dryer, milling, lubrication, and ultimately tableting.
Immediately after the HSWG stage, the dynamic, bulk, and shear properties of the wet granules were gauged using an FT4 Powder Rheometer® (Freeman Technology, UK).
The outcomes of the initial phase (Figure 1) reveal that the Basic Flowability Energy (BFE) of the wet granules can be predicted based on knowledge of process parameters, specifically the Solid-to-Water Ratio and Impeller Speed. Adjusting these parameters enables operators to achieve a desired BFE value.
A subsequent inquiry delved into understanding how granule properties influenced the Critical Quality Attributes (CQAs) of the resulting tablets.
Tablet hardness hinges on die fill depth, blend permeability, and press pressure, influenced by variations in granule density, flow properties, compressibility, and operating speed, among other factors.
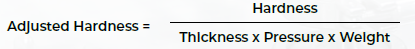
An adjusted measure of tablet hardness was adopted to isolate the relationship between granule properties and tablet quality to compensate for differences in process parameters.
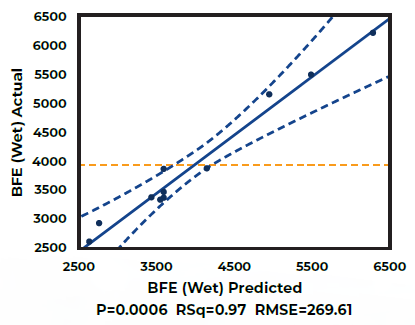
Figure 1. Actual v Predicted Basic Flowability Energy (BFE) values of Wet Granules. Image Credit: Freeman Technology
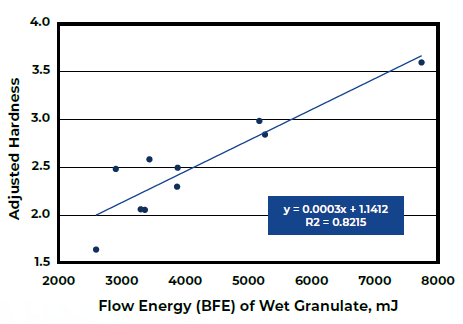
Figure 2. Tablet Hardness with respect to BFE of Wet Granules. Image Credit: Freeman Technology
Analysis of the tableting data (Figure 2) reveals a robust connection between the BFE of the wet granules and Adjusted Hardness. The amalgamation of these two studies illustrates how achieving Critical Quality Attributes (CQAs) of tablets can be realized through the control of Critical Process Parameters.
Prior studies have also demonstrated the link between process variables and CQAs, showcasing the applicability of this approach to a continuous wet granulation process.2
Developing A Design Space to Achieve CQA
Each phase of the process, from granulation to compression, must operate efficiently to yield a high-quality product. Uncontrolled variations at any stage in the manufacturing process can lead to product failures and operational interruptions.
A well-defined Design Space equips operators with the capability to adjust settings, ensuring consistent quality. In this instance, the Basic Flowability Energy (BFE) of wet granules has demonstrated a direct connection to tablet quality.
With a thorough comprehension of relevant material properties and Critical Process Parameters, employing a Quality by Design (QbD)/Design Space approach to batch granulation and tablet manufacturing becomes feasible.
This approach transforms potentially troublesome variations upstream into opportunities for optimizing both process performance and product quality.
References and Further Reading
- T. Freeman, P. Kishinevskaya, J. Huang, M. Moshgbar, John Yin, Evaluating the Design Space for the Batch Manufacture of an OTC Medicine, Freeman Technology, Pfizer Inc.
- T. Freeman, A. Birkmire & B. Armstrong, A QbD Approach to Continuous Tablet Manufacture, Procedia Engineering, 102 (2015), 443-449.

This information has been sourced, reviewed and adapted from materials provided by Freeman Technology.
For more information on this source, please visit Freeman Technology.