Per- and polyfluoroalkyl substances (PFAS) are used in a wide variety of industries, playing a vital role in several technologies essential for modern life.1
One of the most notable applications is in semiconductor manufacturing, where PFAS are used to produce components that are essential to the semiconductor fabrication processes.
Due to growing concern and regulation regarding PFAS emissions and their impact on health and the environment, routine monitoring of PFAS sources has become increasingly important to efforts to reduce the burden of PFAS worldwide.2
Experimental Setup
The experimental setup for thermal release of PFAS from PFA tubing, which is commonly employed due to its unique chemical properties, is shown in Figure 1.
Approximately 5 g of PFA tube was placed within a heated stainless-steel oven and flushed continuously with 2 liters of UHP nitrogen gas per minute. The oven was directly connected to the inlet of a chemical ionization time-of-flight mass spectrometer (CI-TOFMS) and the temperature was linearly increased from 100 °C to 330 °C at a rate of approximately 2 °C per minute.

Figure 1. Experimental setup used in this study. Image Credit: TOFWERK
The emission generated was detected using the rapid polarity switching capability of the TOFWERK Aim Reactor coupled to the Vocus B2, to capture emission by online measurement of positive and negative analyte ions.
Results
All PFAS compounds of interest were detected as the either parent molecule clustered with iodide reagent ions or its deprotonated anion.3 Compounds were identified based on their chemical formula by precise measurement of mass-to-charge ratio and isotopic patterns achieved by fragmentation-free ionization.
A time series of representative PFAS compounds, including PFBA, PFHxA and PFOA (blue trace), is shown in Figure 2 (top). The signal started to increase at 150 °C and continued to grow until ~250 °C. The signal decreased at higher temperatures, indicating that decomposition mechanisms began to play a more dominant role.
As the emitted PFAS compounds decayed, known fluorinated degradation products such as hydrofluoric acid and TFA (red trace) were observed, as well as non-fluorinated compounds including fulminic acid (HCNO) (black), as shown in Figure 2. Similar observations have been reported previously.4

Figure 2. Time series of some compounds emitted from the material as a function of oven temperature (top) and mass defect plots showing total emissions at three temperature points: (A) 100 °C, (B) 250 °C and (C) 330 °C. Image Credit: TOFWERK
Figure 2 (bottom) displays a mass defect plot, showing the total material emissions at three different temperature points. Minimal PFAS emissions were detected at the beginning of the heating process (~100 °C). Increased PFAS emissions were recorded at ~250 °C, including long chain perfluoroalkyl carboxylic acids (PFCAs) with carbon chain lengths of 9 – 18.
At temperatures above 250 °C, elevated levels of various fluorinated and non-fluorinated compounds were detected, the latter being characteristic of various plasticizer products. Volatile hydrocarbon emissions remained low at all temperatures studied, as measured by benzene cations.
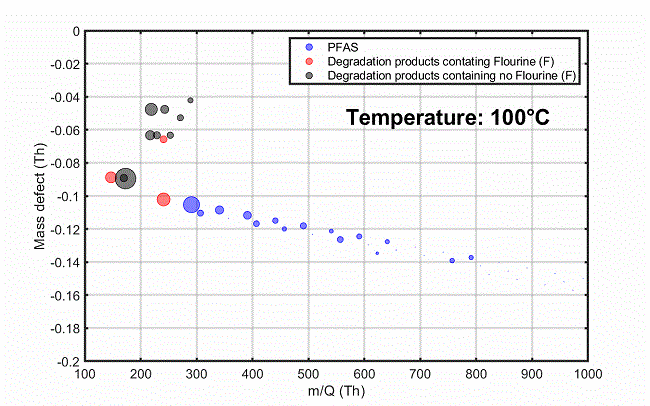
Figure 3. Evolution of material emissions depicted as a function of temperature. Image Credit: TOFWERK
Conclusion
The Vocus B Series with iodide reagent ions has proven valuable for real-time monitoring of PFAS emissions from PFA tubing in the gas phase. While hydrocarbon emissions of PFA are extremely low, the versatility of the Vocus B series makes the instrument invaluable for other material off-gassing applications.
To fine-tune processes and guarantee compliance with regulatory standards, comprehensive emissions and thermal degradation information is essential, particularly for high-performance materials exposed to a variety of environmental conditions and process.
Acknowledgments
Produced from materials originally authored by Priyanka Bansal, Felipe Lopez-Hilfiker and Katie Schmidt from TOFWERK.
References and Further Reading
- Gaines L. G. T. (2022). Historical and current usage of per- and polyfluoroalkyl substances (PFAS): A literature review. American Journal of Industrial Medicine, 66, pp.353-378. https://doi.org/10.1002/ajim.23362
- Panieri E., et al. (2022). PFAS Molecules: A major concern for the human health and the environment. Toxics, 10, 44. https://doi.org/10.3390/toxics10020044
- Bowers, B. B., et al. (2023). Evaluation of iodide chemical ionization mass spectrometry for gas and aerosol-phase per- and polyfluoroalkyl substances (PFAS) analysis. Environmental Science: Processes & Impacts, 25, pp.277-287. https://doi.org/10.1039/D2EM00275B
- Mattila, J. M., et al. (2024). Characterizing volatile emissions and combustion byproducts from aqueous film-forming foams using online chemical ionization mass spectrometry. Environmental Science & Technology, 58, pp.3942-3952. https://doi.org/10.1021/acs.est.3c09255

This information has been sourced, reviewed and adapted from materials provided by TOFWERK.
For more information on this source, please visit TOFWERK.