|
Atomic force microscopy (AFM) provides the ability to perform three-dimensional measurements of surface structures at nanometer-to-subangstrom resolution in ambient and liquid environments. These capabilities have led to ground-breaking life sciences advances in the investigation of DNA, proteins, and cells. In particular, pharmaceutical research involves a number of applications that are rapidly benefiting from AFM, both as a standalone technique and as a powerful complement to the other common analytical techniques currently available.
This application note examines how AFM offers the unique capability of direct, individual investigation of gene delivery vehicles at high resolution in a hydrated state.
AFM History and Methods
AFM is the most commonly used form of the scanning probe microscopy (SPM) family of techniques. The origin of SPM began with the development of the scanning tunneling microscope (STM) in 1982 by researchers at IBM, Zurich.
The ability of the STM to resolve atomic structure on a sample surface earned the inventors the Nobel Prize in 1986. However, the STM can only be applied to conductive or semiconductive specimens. To broaden this type of microscopy to the study of insulators, the atomic force microscope was developed in collaboration between IBM and Stanford University in 1986.
AFM is performed by scanning a sharp tip on the end of a flexible cantilever across a sample surface, while maintaining a small, constant force (see Figure 1).
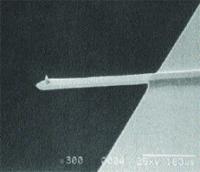
Figure 1. SEM image (300X magnification) of an integrated single crystal silicon cantilever and tip with an end radius of 5nm to 10nm.
The tips typically have an end radius of 5nm to 10nm, although this can vary depending on tip type. The scanning motion is conducted by a piezoelectric tube scanner that scans the tip over the sample in a raster pattern (see Figure 2).

Figure 2. Schematic of the major components of an atomic force microscope, showing the feedback loop for TappingMode operation.
TappingMode and Contact Mode AFM
The tip-sample interaction is monitored by reflecting a laser off the back of the cantilever onto a split-photodiode detector. The two most commonly used modes of operation are contact mode AFM and TappingMode™ AFM, which can be conducted in both air and liquid environments.
In contact mode AFM, a constant cantilever deflection is maintained by a feedback loop that moves the scanner vertically (Z) at each lateral (X,Y) data point to form the topographic image.
By maintaining a constant deflection during scanning, a constant vertical force is maintained between the tip and sample. Although contact mode has proven useful for a wide range of applications, it is not as effective on relatively soft samples. On the other hand, TappingMode AFM consists of oscillating the cantilever at its resonance frequency (typically ~300kHz) and scanning across the surface with a constant, damped amplitude. The feedback loop maintains a constant rootmean-square (RMS) amplitude by moving the scanner vertically during scanning, which correspondingly maintains a constant applied force to form a topographic image. The advantage of TappingMode is that it typically operates with a lower vertical force than that possible with contact mode, and it eliminates the lateral, shear forces that can damage some samples. Thus, TappingMode has become the preferred technique for imaging soft, fragile, adhesive, and particulate surfaces.
AFM in Gene Delivery Research
Gene therapy has been gaining momentum as an effective method for treating genetic-based diseases.
However, one of the primary obstacles facing this form of treatment is in the delivery of the condensed genetic material to its intended target. There are two common methods of packing DNA for gene delivery: viral and nonviral.
Though the viral-mediated mode of gene delivery is currently the most common, it can be problematic due to the activation of a patient’s immunological response, which may eliminate the gene delivery vehicle before use and produce other health problems. To avoid these complications, nonviral vehicles fabricated out of liposomes or polymers are increasingly being used to encapsulate the DNA or other drug related materials. AFM has successfully improved the development of such gene delivery vehicles by providing a greater understanding of the process of DNA condensation. Figure 3 shows nonviral DNA condensates that were formed with different charge relationships.
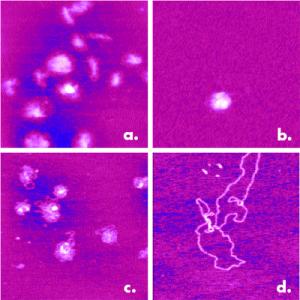
Figure 3. Four different condensed states of DNA from a study of nonviral gene delivery vehicles: a) Condensed negatively charged on NiCl2-treated mica, b) Condensed negatively charged with 0.2 mM NiCl2, c) Condensed positively charged, d) Noncondensed. Depending on the formation mechanism, the condensates are tightly packed or slightly unraveled. 1μm scans.
Variables in the formation process result in either positive or negative charged condensates, producing varying degrees of DNA packing.
AFM offers a distinct advantage over other methods for investigating the DNA condensates. One of the greatest advantages is AFM ability to view the structure of the delivery vehicle in its hydrated state, as it would appear in use. In addition, with AFM nanometer-scale resolution, researchers can easily image the DNA strands and see how they react and condense with a particular polymer or liposome. No other technique allows direct investigation of individual vehicles at high resolution in a hydrated state. For instance, there are various particle-sizing techniques that produce a size distribution over a very large number of condensates, but they cannot be applied to an individual condensate. One of the most common techniques currently used is electron microscopy (EM), which offers the high resolution needed to view individual condensates, but requires significant sample preparation time and requires the specimen to be dried out in a vacuum environment. Since, drying out the gene delivery vehicles may change their structure, the results are, at best, less useful. At worst, these results may be misleading and result in ineffective gene delivery vehicles.
Generally, AFM is applied to gene delivery vehicles that are in a solution. They are injected into the fluid cell where they attach to a substrate, typically mica. The vehicles are held on the mica surface by charge. Positively charged condensates are naturally attracted to the negative charge of the mica surface, and negatively charged condensates can be attracted to the negatively charged mica by either placing a divalent cation in the solution, or by coating the mica with a silane to form AP-mica. AFM imaging is then conducted in solution via the TappingMode technique. Though simple to prepare and perform, AFM is thus able to provide very high-resolution on individual DNA condensates.
Gene Delivery Studies Using AFM
There have been a number of published AFM-based gene delivery studies. Dunlap and coworkers studied variations in the condensation mechanisms of supercoiled plasmid DNA 5–7kb in length with a cationic lipid, lipospermine (dioctadecylamidoglycylspermine or DOGS), and a cationic polymer, polyethylenimine (PEI). The resulting condensates were imaged by TappingMode in 15mM NaCl solution.
For both DOGS and PEI, folded DNA loops radiating from central cores were evident, indicating condensation by packing folded loops of DNA. In Figure 4, bundles of DNA can be clearly seen along with the polymer globules. This partial condensate has formed a toroidal structure from the circularization of an oblong condensate with multiple condensation nodes. By varying the concentration of DOGS and PEI, the conditions for complete condensation were also investigated. Complete PEI condensates were found to be 20 to 40 nanometers in diameter, whereas complete DOGS condensates were found to be 50 to 70 nanometers. This suggests that PEI may be a more effective condensing agent than the DOGS. The difference in size and morphology may also affect their efficiency as a transfection agent.
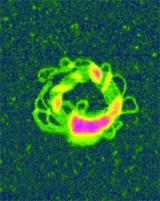
Figure 4. Nonviral gene delivery with condensed DNA and a polymer. Orangeviolet globules of polyethylenimine (PEI), a cationic polymer, stabilized circular bundles of yellow-green DNA loops in a five kilobase plasmid. Unfixed molecules were imaged in TappingMode in 15mM salt solution.
AFM has also been used to image dynamic processes in situ to gain a better understanding of the formation mechanisms associated with DNA condensation. Condensation of pegylated poly(amidoamine) with DNA has been observed in aqueous solution in real-time. The overall positive charge of the polymer-DNA condensate was used to electrostatically hold the condensates to the negatively charged mica. However, it was not held so rigidly as to prevent movement of the condensates during their formation.
Imaging of the condensates in solution indicated the presence of toroidal and rod-like condensate structures. In Figure 5, the formation of a toroidal condensate can be seen over a timespan of 35 minutes. From these images, the toroidal structure appears to be formed by joining the ends of a rod-like condensate.

Figure 5. Formation of toroidal DNA-polymer condensate over 35 minute time-span. Scale bar = 200nm.
Further investigations have shown the existence of rod-like and toroidal structures in a state of dynamic equilibrium, with the condensates changing from rod-like to toroidal, and toroidal to rod-like structures. Studying the dynamic processes in real-time makes it possible to gain a better understanding of the kinetics of the condensation process, which could lead to significant improvements in gene delivery.
Conclusion
AFM has demonstrated success in studying nanoscale, in situ DNA structures. This has an obvious relevance to efforts to develop more effective gene delivery vehicles. Researchers are utilizing the many benefits of AFM—high resolution, simplified sample preparation, real-time investigation, non-destructive imaging, and the ability to perform in liquid—to investigate DNA condensation mechanisms and various gene-packaging materials. Although the examples discussed above are just a sampling of the work that has been conducted with atomic force microscopes in gene delivery studies, they indicate how important the AFM is to the future of gene therapy. The unique benefits of AFM will almost certainly play a crucial role in gene therapy development.
|