Researchers seeking to enhance fuel-cell catalysts made of nanoparticles have discovered that the catalytic activity of nanoparticles can be correlated with the type and count of their surface facets.
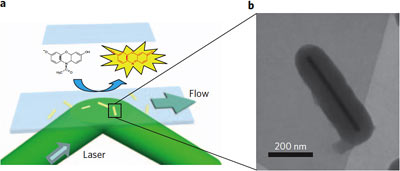 When Amplex Red connects with a gold catalyst the structure is changed to make a fluorescent molecule that immediately emits a flash of light, showing where the catalytic event took place. Right, electron microphoto of a single gold nanorod, encased in a poirus silica shell. The shell keeps rods from clumping together and allows experimenters to use heat to clean away a coating that forms when the rods are created. (Provided/Chen Lab)
When Amplex Red connects with a gold catalyst the structure is changed to make a fluorescent molecule that immediately emits a flash of light, showing where the catalytic event took place. Right, electron microphoto of a single gold nanorod, encased in a poirus silica shell. The shell keeps rods from clumping together and allows experimenters to use heat to clean away a coating that forms when the rods are created. (Provided/Chen Lab)
However, according to Peng Chen from the Cornell University, the surface defects on the facets of the nanoparticles dominate their catalytic activity. The research findings have been reported in the online edition of Nature Nanotechnology.
Chen's research team investigated the catalytic activity of gold nanorods with a length of up to 700 nm, which allowed the team to study the difference in the catalytic activity over a single facet. Gold functions as a catalyst to help in the production of a fluorescent called resorufin from a chemical known as Amplex Red.
Every time a catalytic event happens, a light flash is emitted by the newly formed resorufin molecule. This flash light is determined by a digital camera integrated with a microscope and an additional computer processing averages the brightness of the flash light to pinpoint the actual incident to within a few nanometers. This technique is called as ‘super-resolution microscopy’ by the research team.
The research team filmed a movie at a rate of one frame per 25 ms after submerging a nanorod field with an Amplex Red solution. The team observed more number of catalytic occurrences nearby the middle of a nanorod, reducing toward the ends and increased again at the ends. It also discovered that the amount of catalytic activity varied between the nanorods even that have similar type of facets.
Based on the results, the research team proposed that areas that had more surface defects demonstrated more activity. Surface facet information alone is not enough to estimate a catalytic activity and surface defects are also capable of playing a major role. Chen concluded that these findings are also applicable to other type of catalysts that are utilized for pollution remediation and in fuel cells.