A research team led by Chongmin Wang, a materials scientist at the Department of Energy's Pacific Northwest National Laboratory (PNNL), has conducted a study on a new silicon-carbon nanocomposite electrode material.
 This composite image shows a silicon-carbon nanofiber electrode before (left) and after (right) being charged with lithium ions
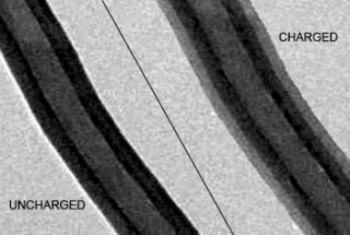
This composite image shows a silicon-carbon nanofiber electrode before (left) and after (right) being charged with lithium ions
The study describes the function of the material and also reveals the reasons for high performance when compared to individual silicon material. The silicon-carbon nanofiber electrodes can lead to the development of cheaper, longer-lasting rechargeable lithium batteries for electric vehicles (EVs), as their electrical capacity is five times greater than conventional electrodes.
The use of silicon as a battery material has both pros and cons. It has an excellent energy storage capacity, therefore it can retain a large amount of charge. However, the main issue is that the silicon swells up when the battery is charged. This can lead to bursting of batteries. To overcome the problem, Wang and his team planned to test nano-sized electrodes, which include carbon nanofibers with silicon coating.
At first, scientists tested the quantity of lithium that can be retained by the electrodes and the amount of time it lasted by placing the electrodes in a half-cell, which is a small testing battery. The electrodes continued to have an excellent storage capacity of approximately 1000 mAh/g of battery material, after 100 charge-discharge cycles. The capacity is five to ten times greater than the capacity of conventional battery electrodes.
The team has also determined the ability of the electrodes to withstand repeated stretching using a transmission electron microscope. A specially designed tiny battery was observed under the microscope to study the function of the electrode. Researchers have found that the silicon layer has expanded up to 30% as the lithium ions flow into it. However, the carbon support along with the silicon's unique quality resulted in uniform swelling of the layer. In contrast, the layer swells unevenly in case of silicon alone. PNNL team has also examined the crystallization of the lithium and silicon layer in the microscope. Researchers have been able to observe the advance in the crystallization process with the lithium to silicon ratio reaching 15:4.