Apr 29 2015
Researchers have used advanced 3D imaging to show how Li-ion batteries explode they are overheated. The study was a collaborative effort of University College of London (UCL), the National Physical Laboratory, ESRF The European Synchrotron, and Imperial College London.
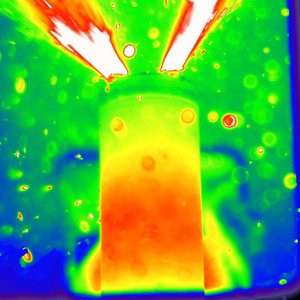 Battery showing thermal runaway during thermal abuse tests (Credit: Donal Finegan, UCL)
Battery showing thermal runaway during thermal abuse tests (Credit: Donal Finegan, UCL)
In order to improve the design of the Li-ion batteries and make them safer, it is important to understand how these batteries overheat and explode. Rechargeable batteries are important in today’s modern living. Each year, a countless number of these batteries are produced to power cars, planes, laptops and mobile phones.
While battery failure is not a common issue, the US Federal Aviation Administration tests revealed that when batteries are overheated, it can cause a major fire. In view of these test results, three airlines had made an announcement that lithium-ion batteries will no longer be carried in their cargo planes.
For the first time, the new study shows how damage to the internal structure of batteries arises in real-time, and indicates how this structural damage can extend to the adjacent batteries.
"We combined high energy synchrotron X-rays and thermal imaging to map changes to the internal structure and external temperature of two types of Li-ion batteries as we exposed them to extreme levels of heat. We needed exceptionally high speed imaging to capture ‘thermal runaway’ – where the battery overheats and can ignite. This was achieved at the ESRF beamline ID15A where 3D images can be captured in fractions of a second thanks to the very high photon flux and high speed imaging detector," said the first author, UCL PhD student Donal Finegan.
In the past, the battery’s failure mechanism was examined through X-ray computed tomography and investigated with stationary images. Changes in the batteries were also monitored under standard operating conditions.
The researchers studied the effects of the formation of gas pockets, venting, and rising temperatures on the layers within two different commercially available Li-ion batteries by subjecting the battery shells to more than 250°C.
The battery that had an internal support was not significantly damaged up until the beginning of thermal runaway. At this point, the copper present within the cell melted, indicating temperatures of about 1000°C. This heat extended from the inside of the battery to the outside, resulting in thermal runaway.
On the contrary, the battery that did not have an internal support exploded, which made the whole cap of the battery to separate, ejecting its contents. Before thermal runaway, the closely packed core collapsed, causing damage to adjacent objects and increasing the risk of internal short circuits.
"Although we only studied two commercial batteries, our results show how useful our method is in tracking battery damage in 3D and in real-time. The destruction we saw is very unlikely to happen under normal conditions as we pushed the batteries a long way to make them fail by exposing them to conditions well outside the recommended safe operating window. This was crucial for us to better understand how battery failure initiates and spreads. Hopefully from using our method, the design of safety features of batteries can be evaluated and improved," said corresponding author, Dr Paul Shearing (UCL Chemical Engineering).
The researchers intend to study these effects in a larger sample size of batteries, with a particular focus on the microscopic-level changes that contribute to extensive battery failure.
The National Physical Laboratory, Royal Academy of Engineering, and Engineering and Physical Sciences Research Council (EPSRC) funded the study. The ESRF provided the ‘beam time’ to perform these experiments.
The study results have been featured in Nature Communications.