Oct 15 2015
The method involves using a new electrolytic solvent that ensures more lithium is available for current generation.
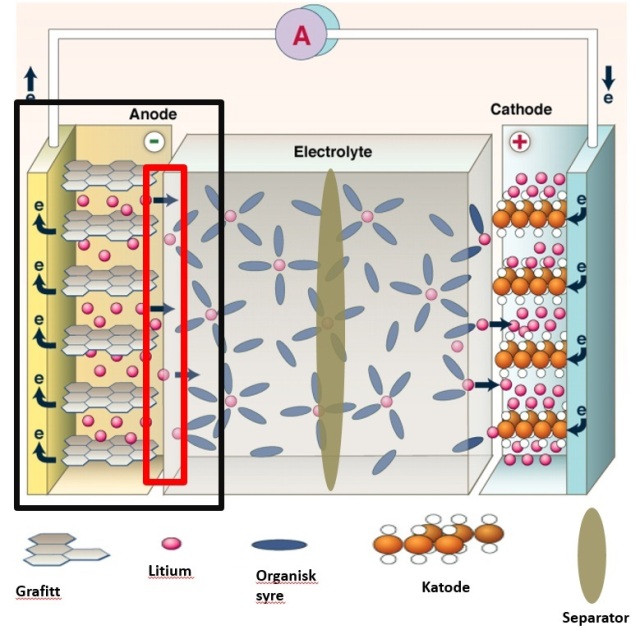 Here is where the SEI layer is created. Lithium that is in the layer cannot be used for charging or powering the battery. The goal is to limit the amount of lithium that is bound up in this layer. Key: Grafitt= Graphite; Litium= Lithium; Organisk syre: Organic acid; Katode= Cathode; Separator= Separator Illustration: Paul Scherrer Institute
Here is where the SEI layer is created. Lithium that is in the layer cannot be used for charging or powering the battery. The goal is to limit the amount of lithium that is bound up in this layer. Key: Grafitt= Graphite; Litium= Lithium; Organisk syre: Organic acid; Katode= Cathode; Separator= Separator Illustration: Paul Scherrer Institute
Lithium batteries are widely used in a myriad of electronic devices. They can be found in almost all portable devices including mobile phones, laptops, cars and most other vehicles. However, their capacity begins to decrease when they are used repeatedly.
The batteries currently being used can operate at temperatures above -10°C. However, a new method has been developed that will allow batteries to function at temperatures as low as -50°C.
Lithium batteries are composed of a negative electrode and a positive electrode. The electrodes are conductive and each possesses an opposite charge. In the lithium batteries the cathode, the negative electrode, consists of graphite. Whereas the anode, the positive electrode, consist of lithium salt dissolved in an organic solvent which acts as an electrolyte. Lithium ions are carried by the electrolyte between the two electrodes which results in a flow of current. Research is currently being conducted to modify the electrolyte’s composition in an attempt to enhance the batteries.
When a lithium battery is initially charged a thin solid electrolyte interphase (SEI) film forms on the graphite anode’s surface. This film is made up of both organic and inorganic lithium compounds meaning it has a complex chemical structure. The thin film forms due to the reaction that takes place between the anode and the electrolyte. It plays an important role in determining the capacity, thermal stability and lifetime of a battery.
This film is an essential part of the battery.
Prof. Ann Mari Svensson - NTNU
Lithium that gets bound in the film during the first charge is unavailable to take part in the current inducing processes that occur when the electrodes are charged. This leads to a decrease in the batteries capacity.
Once the battery is assembled, we cannot add more lithium to the cell, and therefore limiting the loss of available lithium in the cell is of prime importance for long lasting batteries.
Ahmet Oguz Tezel - NTNU
Tezel has submitted his PhD on SEI film formation and the way that electrolyte composition influences film formation. He has attempted to increase the life span and capacity of lithium batteries at low temperatures by modifying the electrolyte. Additionally, he has also developed a preparatory treatment that minimizes the loss of lithium during the process of SEI film formation. This also enables more lithium to take part in current formation.
Electrolytes containing ethylene carbonate (EC) possess a high melting temperature which prevents them from functioning at temperatures as low as -10°C. However batteries that contain propylene carbonate (PC) can function at temperatures as low was -50°C.
We think that we found a way to substitute EC for PC, however we haven’t confirmed this yet .But our research suggests how it might be realized.
Ahmet Oguz Tezel - NTNU
Professor Ann Mari Svensson and Professor Svein Sunde at the Department of Materials Science and Engineering at the Norwegian University of Science and Technology (NTNU) supervised Tezel’s work. He also works at Graphene Batteries AS.