Mar 23 2016
For the first time, polymer scientists from the University of Massachusetts Amherst have discovered the factors that control the final size and shape of chiral filament bundles that self-assemble into different architectures. The research team, which included Greg Grason, Isaac Bruss and Douglas Hall, along with Justin Barone from Virginia Tech, reported the experimental results that support their novel model. The study has been reported in the current issue of the Nature Materials journal.
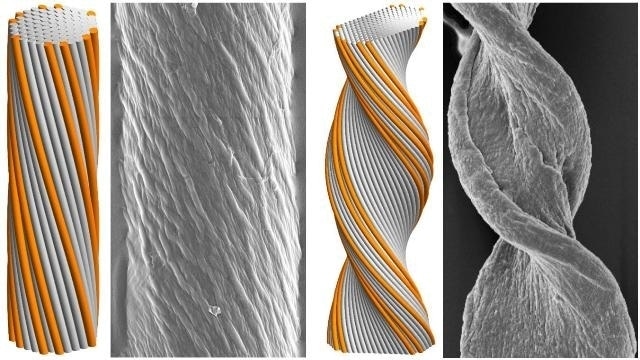 Cyindrical (left) and tape-like (right) twisted filament bundles: model morphologies (simulated assemblies) and experimental observations (amyloid fibers). Greg Grason and colleagues have for the first time identified key factors that govern the final morphology of self-assembling chiral filament bundles. Courtesy UMass Amherst/Greg Grason
Cyindrical (left) and tape-like (right) twisted filament bundles: model morphologies (simulated assemblies) and experimental observations (amyloid fibers). Greg Grason and colleagues have for the first time identified key factors that govern the final morphology of self-assembling chiral filament bundles. Courtesy UMass Amherst/Greg Grason
Grason explained that at the molecular level, the chiral filament bundles are self-twisting, multi-stranded, yarn-like structures. Amyloid fibers are one example of chiral filament bundles. These fibers are assemblies of misfolded proteins that are associated with Parkinson’s and Alzheimer’s diseases. A large number of proteins, including collagen and sickle-hemoglobin proteins, assume this shape. Collagen is a protein that is abundantly present in the body, while sickle-hemoglobin proteins are present in sickle-cell anemia. However, it is still unclear how these proteins achieve their final morphology.
Bruss and Grason carried out a previous study, which described the development of filament bundles that look like a cable or rope. Grason presented this work at Virginia Tech, where he was approached by biological systems engineer Justin Barone regarding the geometric makeup of amyloid fibers. Barone asked why the shapes of amyloid fibers, which he was studying, appeared flat and tape-like in some conditions and cylindrical in other conditions.
Justin’s questions about the shape of amyloid fibers set us on the track to figure out how fibers form from many copies of identical filaments and know what shapes to be. Since filaments attract one another, understanding what makes fibers grow fatter is not so hard. The challenge is to understand what makes the process stop at certain sizes, and why a fiber sometimes grows larger in one direction than the other, leading to different cross-sectional shapes. Based on our new model, we have new design rules for controlling the size and shape of ‘self-spinning’ nano-fiber materials used in applications such as soft-gel scaffolds that can be deployed in filters, sensor patches or any place where you need material architectures with tunable mechanics and size scales.
Greg Grason, Polymer Scientist, Virginia Tech
Many structures in the human body consist of collagen bundles or other types of protein filaments, Grason explains.
The bundles in your eye are small and more uniform because the cornea has to be transparent, compared to fibers that make up your tendons, which have to be thicker and stronger. These different tissues are formed from basically the same building blocks, yet they assemble into different architectures. We wanted to develop a physical model of what governs the structure of protein fibers and other fiber-forming systems. How do they self-organize and what determines their size and shape? The basic ingredients are molecular-scale, nano-filaments that stick to one another, forming a structure that looks like rope or cable, made of strands that twist together.
Greg Grason, Polymer Scientist, Virginia Tech
Grason added that it a well-known fact that the screw-like structure of the chiral filaments caused the filaments to coil around one another in the form of bundle assemblies. However, what was unknown was how the screw-like structure along the length governs the strands’ lateral distribution in the fiber.
Combining mathematical and geometric models with computational simulations, the team identified that the amount of strands involved predicts the relative strength of the final morphology is, and whether the protein filaments of this structure will assume a ribbon-like shape or a cylindrical shape in cross section.
According to Grason, the major insight that was made earlier by his team was to demonstrate that filament twisting occurring in the bundles ‘frustrates’ the layers between adjoining filaments, making it unfeasible to uniformly space the filaments in a cross section. The basis of this frustration is that it results in a feedback mechanism between the lateral shape and the twist pattern of the bundle.
We now have a model that explains how the number of strands underlies morphology selection. A smaller number of strands allows them to keep the right distance from their neighbors, so even though there is a twist, if the twist is not too big and the strand number not too big, the structure doesn’t get too crowded and keeps a cylindrical cross section. But once the number of strands gets larger, outer strands get too close for comfort and a tape-like or twisted ribbon structure will emerge.For the first time, we are able to predict that the frustration will lead to new shape transitions. For relatively narrow and weakly twisted bundles, the cost of fewer contacts at the sides of the bundle favors a cylindrical shape. But above a critical size, the cost of the frustration causes the morphology to change dramatically, leading to bundle shapes that are very anisotropic, much wider in one dimension and thinner in the other.
Greg Grason, Polymer Scientist, Virginia Tech
To verify the new predictions, Bruss, now at the University of Michigan, and Hall worked together to develop and use a simulation model, to demonstrate how the pattern of filament twisting determines how fat these assemblies can be, how large they will grow, and what kind of morphology their cross sections will exhibit. The team observed that both experimental and simulated amyloid fibers may be divided as tape-forming or cylinder-forming as per a relatively easy combination of geometric and molecular parameters of the assembly.
To start out with complex and unexplained observations, then to design the model that let others run the simulations to show it was true, then to bring it all the way back to confirm in the experimental data was particularly satisfying. It doesn’t always happen this way.
Greg Grason, Polymer Scientist, Virginia Tech
The study was funded by the Alfred P. Sloan Foundation and a National Science Foundation CAREER award.