Aug 3 2016
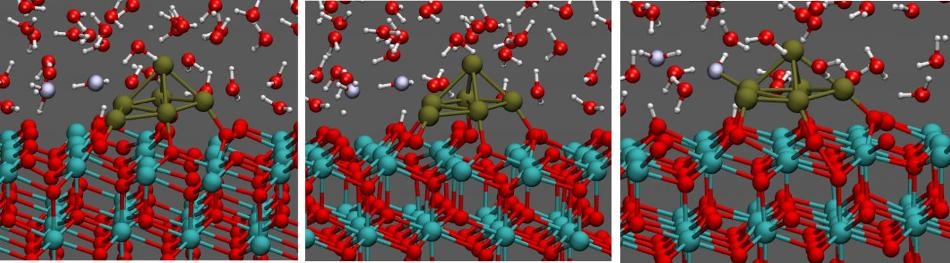 Water on catalyst. (Credit: CNR IOM/SISSA)
Water on catalyst. (Credit: CNR IOM/SISSA)
A reaction resembling a type proton pinball game enables a thin layer of moisture present on the surface of catalysts to enhance the effectiveness of the fuel cells.
These cells are devices that convert chemical energy - such as a fuel like hydrogen - directly into electricity without discharging greenhouse gases in emissions. IOM CNR/SISSA coordinated the study.
The function of the fuel cells is to convert chemical energy into electricity through a chemical reaction. Fuel cells, such as hydrogen, can be used without giving out CO2 into the atmosphere when this technology is well established.
The chemical reaction in the fuel cell is facilitated by a catalyst, which is usually platinum nanoparticles scattered on the surface of a reactive and durable material, such as cerium oxide.
Before the present study, the active areas of these catalysts were examined under perfect conditions, at extremely low pressures and temperatures, removing moisture and dirt that could be detected in the devices under normal working conditions.
However, Stefano Fabris, a Physicist at the International School for Advanced Studies (SISSA) of Trieste and CNRIOM Istituto Officina dei Materiali, and colleagues, wanted to analyze a system in realistic conditions, in this situation adding a thin water layer on the catalyst.
Some of the remarkable discoveries made by the team highlight the fact that the moisture, instead of making the entire process less effective, provide a boost to the atoms in transit, majorly enhancing the system’s complete efficiency. The Journal of the American Chemical Society published this study coordinated by Fabris.
The research carried out by Fabris and team is based on computer simulations.
This is a not an insignificant aspect, because traditional experimental techniques do not allow us to obtain detailed information about what happens at the interface between the surface of the catalyst and a liquid such as water. In this way, the atomic layers that separate the solid and the water remain a largely unexplored world, as difficult to measure as the core of a planet. The pressure and temperature conditions prevent a direct view at the experimental level. We must therefore find other ways to investigate this kind of phenomena, such as using these numerical simulations.
Stefano Fabris, Physicist, SISSA
Chain Reaction
The team reconstructed the physical system in detail, precisely where the surface of the catalyst touches one or more layers of water molecules. The team further observed the system’s evolution in real time. "First, we noticed that the water in contact with the catalyst breaks down, in part, into hydrogen ions, or protons, and hydroxide ions (OH-).
Matteo Farnesi Camellone, CNR-IOM (Istituto Officina dei Materiali) researcher and first author of the work, stated that this was not completely unexpected and that an effect like this one could have been imagined a priori.
"The really interesting part happens after this breakdown," says Farnesi Camellone.
The existence of a specific number of hydroxide ions and protons on the surface results in the occurrence of a proton chain: "a sort of pinball game where the OHgroups pass a free proton back and forth incessantly, binding it and releasing it. In the process water molecules form and break up continuously, while the protons continue to bounce and travel long distances along the surface."
Positive consequences for the catalytic process are obtained. “All of this movement helps transport molecules between the active zones of the material. We measured increases in transport and release speed several times, the efficiency of the catalyst actually improves."
This is the first time the catalyst has been studied with water present. Our study, besides showing that the process is favored by moisture, goes beyond to explain what happens in the material in detail, which is important knowledge for designing better fuel cells.
Stefano Fabris, Physicist, SISSA