Nov 4 2016
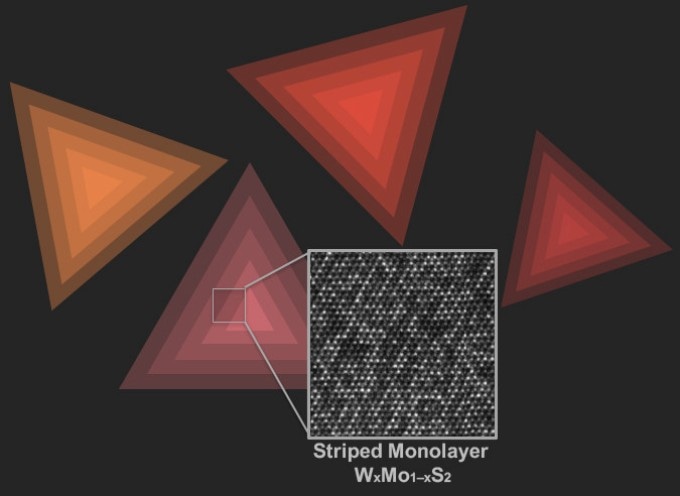 An electron microscopy image of ordered atoms of tungsten (W) and molybdenum (Mo) against artistic representations of triangular single layer flakes of WxMo1–xS2 on a substrate. Credit: Amin Azizi and Andrea Kohler/ Penn State
An electron microscopy image of ordered atoms of tungsten (W) and molybdenum (Mo) against artistic representations of triangular single layer flakes of WxMo1–xS2 on a substrate. Credit: Amin Azizi and Andrea Kohler/ Penn State
Scientists at Penn State believe that the discovery of atomic chains in a two-dimensional crystal could help them to find a way to handle the direction of materials properties in both two- and three-dimensional crystals. This could have implications in next-generation electronics, optoelectronics and sensing applications.
Whether an alloy has an arrangement of atoms that is ordered or a random arrangement, it can have great effects on the properties of a material. Nasim Alem, assistant professor of materials science and engineering, and his colleagues at Penn State have combined scanning transmission electron microscopy (STEM) imaging and simulations in order to calculate the atomic structure of an ordered alloy of tungsten, sulfur, and molybdenum.
They are determined that fluctuations in the quantity of available sulfur were responsible for the formation of either tungsten or molybdenum atomic chains. The research paper was published online in Nano Letters.
We discovered how chains form in a two-dimensional alloy as a result of fluctuations in the amount of a particular precursor, in this case sulfur. Normally, when we combine atoms of different elements, we don't know how to control where the atoms will go. But we have found a mechanism to give order to the atoms, which in turn introduces control of the properties, not only heat transport, as is the case in this work, but also electronic, chemical or magnetic properties in other alloy cases. If you know the mechanism, you can apply it to arrange the atoms in a wide range of alloys in 2D crystals across the periodic table.
Nasim Alem, Assistant Professor, Penn State
The researchers showed, in the case of sulfur, tungsten and molybdenum alloy, that the electronic properties were similar in all directions, but using simulations, they foresee that the thermal transport properties are smaller perpendicular to the stripes or chains.
"We didn't know why this crystal forms an ordered structure, so we worked with my colleague Dr. Vin Crespi to understand the underlying physics that causes order in this crystal," said Alem. "Our calculations show it was the fluctuations in the third element, sulfur, that was determining how the chains formed."
Although the interior of the flake is indifferent to whether molybdenum or tungsten occupies any site in the crystal lattice, the edge of the growing crystal does care: Depending on how much sulfur is available at a given location, the edge will prefer to be either 100 percent molybdenum or 100 percent tungsten. So as the availability of sulfur randomly varies during growth, the system alternately lays down rows of molybdenum or tungsten. We think this may be a general mechanism to create stripe-like structures in 2D materials.
Vincent H. Crespi, Professor, Penn State
The STEM imaging and spectroscopy, produced by Amin Aziz, a Ph.D. candidate in Alem's group and lead author, showed both the fine atomic structure and the electronic properties of the alloy samples.
"When we are able to directly image constitutive atoms of a substance, see how they interact with each other at the atomic level and try to understand the origins of such behaviors, we could potentially create new materials with unusual properties that have never existed," said Azizi,
A team headed by Mauricio Terrones, professor of physics, created these ordered alloy samples by vaporizing powders of all the three elements, termed precursors, under high heat.
Along with Azizi, Terrones, Alem and Crespi, other coauthors on the paper titled "Spontaneous formation of atomically thin stripes in transition metal dichalcogenide monolayers," include Yuanxi Wang, a Ph.D. candidate in Crespi's group, Ke Wang, a staff scientist in the Materials Research Institute who helped with the electron microscopy, Ph.D. candidate Zhong Lin in materials science and Ana Laura Elias, research associate in the Terrones group.
The research work was supported by the National Science Foundation and the U.S. Army Research Office.