Feb 11 2019
A study team led by Hokkaido University in Japan has created a porous material that is extremely stable and changes color when exposed to acid vapor. This is supposed to be the first reported case of a hydrogen-bonded organic framework changing color in reaction to acid. The outcomes are explained in the Journal of the American Chemical Society.
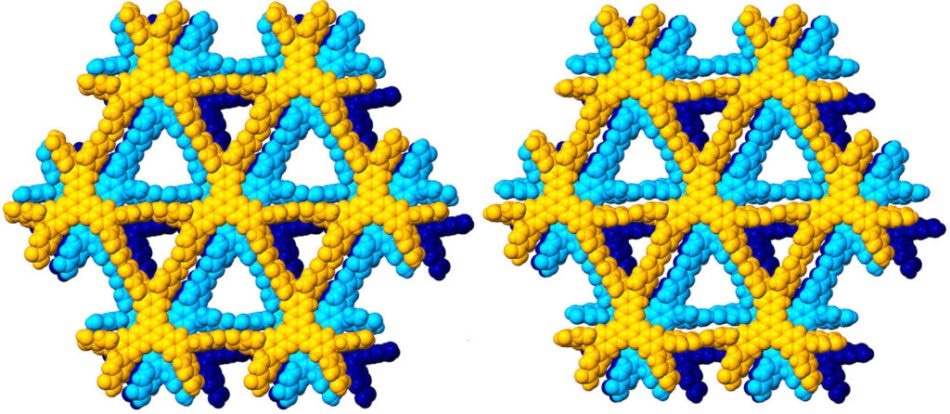 Image of the created hydrogen-bonding organic framework CPHATN-(TCB) (left), and its activated version, CPHATN-1a (right). (Image credit: Hisaki I. et al., Acid Responsive Hydrogen-Bonded Organic Frameworks, Journal of the American Chemical Society, January 7, 2019.)
Image of the created hydrogen-bonding organic framework CPHATN-(TCB) (left), and its activated version, CPHATN-1a (right). (Image credit: Hisaki I. et al., Acid Responsive Hydrogen-Bonded Organic Frameworks, Journal of the American Chemical Society, January 7, 2019.)
Chemists are aiming to create porous materials composed of organic molecules that have structures with distinct openings capable of separating and storing gasses. These materials can also be used in sensors and electronic devices.
Specifically, scientists are examining how to create materials with molecules bonded together by hydrogen bonds, referred to as hydrogen-bonding organic frameworks (HOFs). HOFs are high-crystalline, regenerable, and flexible, making them appealing choices. But they can also be brittle and crumble apart.
Ichiro Hisaki, a chemist at Hokkaido University’s Research Institute for Electronic Science, together with Anderrazzak Douhal, a photophysicist at University of Castilla La Mancha, Spain and their colleagues created a hexagon-shaped framework, called CPHATN-1a, and found a unexpected trait—its color changes from yellow to reddish-brown when exposed to acid vapor or acid solution. When the acid vapor or solution is removed, either by heating or ambient evaporation, the HOF changed back to its original yellow color.
The scientists established that the color change is the result of protons adding onto nitrogen atoms within the compound, which changes the spectrum of light absorbed.
Extra tests exposed that CPHATN-1a is very stable, preserving its porous structure at temperatures of at least 633 K (359 °C). The strong material also endured heated, common organic solvents, including ethanol, chloroform, and water, retaining its structure instead of breaking apart or dissolving.
“The present results would open a door to develop new porous materials with stimuli responsiveness,” the researchers observe. “These could be used in the creation of new sensors or towards the visualization of minute chemical reactions.”
This research was financially supported by a Ministry of Education, Culture, Sports, Science and Technology, Japan Grant-in-Aid for Scientific Research (JP15K04591, JP18H01966), and Ministry of Economy and Competitiveness (MINECO), Spain Projects (MAT2014-57646-P, MAT2017-86532-R).