Feb 22 2019
The latest developments in battery technology—ranging from the design of their cases to the electrochemistry occurring inside them—have led to an increased number of many different electric cars, including Leafs, Teslas, and Volts.
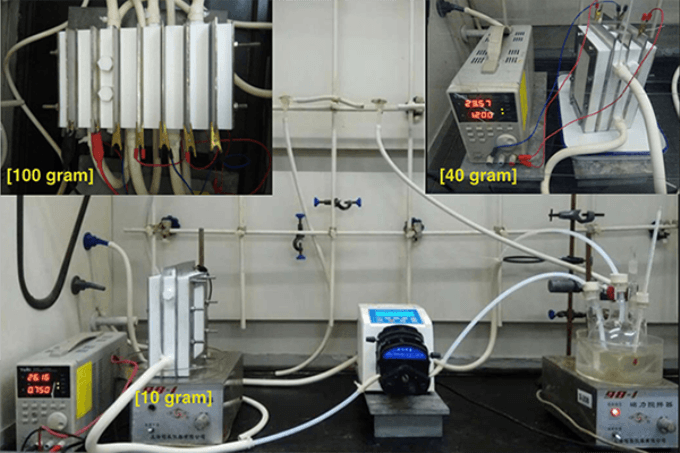 Scientists at Scripps Research, inspired by the refined electrochemistry of these batteries, have developed a battery-like system that allows them to make potential advancements for the manufacturing of medicines. Their system avoids safety risks associated with a type of chemical reaction known as dissolving metal reduction, which is often used to produce compounds used in the manufacturing of medicines. (Image credit: Baran lab)
Scientists at Scripps Research, inspired by the refined electrochemistry of these batteries, have developed a battery-like system that allows them to make potential advancements for the manufacturing of medicines. Their system avoids safety risks associated with a type of chemical reaction known as dissolving metal reduction, which is often used to produce compounds used in the manufacturing of medicines. (Image credit: Baran lab)
Now, a unique, battery-like system has been developed by Scripps Research scientists, who were inspired by the batteries’ refined electrochemistry. This system enables the team to make promising developments in the manufacture of drugs.
The latest technique, reported February 22nd, 2019, in Science, prevents safety risks related to a specific kind of chemical reaction called dissolving metal reduction, which is generally utilized for producing compounds employed in the development of medicines. The researchers’ method would provide significant benefits over existing approaches of chemical manufacturing, but has been considerably sidelined owing to safety considerations, until now.
The same types of batteries we use in our electric cars today were far too dangerous for commercial use a few decades ago, but now they are remarkably safe thanks to advances in chemistry and engineering. By applying some of the same principles that made this new generation of batteries possible, we have developed a method to safely conduct powerfully reductive chemical reactions that have very rarely been used on a large scale because—until now—they were too dangerous or costly.
Phil Baran, PhD, Study Senior Author and the Darlene Shiley Chair, Department of Chemistry, The Scripps Research Institute.
The study has been published in the Science journal.
“This could have a major impact on not only the manufacturing of pharmaceuticals,” added Baran, “but also on the mindset of medicinal chemists who traditionally avoid such chemistry due to safety concerns. This problem was in fact brought to our attention by co-author Michael Collins, a medicinal chemist at Pfizer, for precisely this reason.”
Birch reduction is one among the most intense reactions and also one of the representative examples of this highly reducing chemistry used by chemists to develop innovative molecules. Arthur Birch, an Australian chemist, largely pioneered this reductive reaction in the 1940s. In this reaction, a reactive metal is dissolved in liquid ammonia to exploit ring-shaped molecules that can be applied as the basis for developing many numbers of chemical products, such as drug molecules.
In this process, ammonia or analogous compounds have to be condensed, but these compounds are volatile, toxic, and corrosive and have to be mixed with metals like lithium that have a tendency to burst into flames upon exposure to air. In addition, the process should take place at very cold temperatures, needing specialists and costly equipment.
A compound that was previously under development at Pfizer is an exceptional example of the application of a dissolving metal reduction in pharmaceutical manufacturing. This represents an incredible accomplishment in chemical manufacturing that called for a phenomenal effort. The system to create the compound on a massive scale needed a sufficient amount of gaseous ammonia to fill three Boeing 747 airliners and needs to be performed at –35 °C. The synthetic power of the reaction is a testimony to the efforts Pfizer took to apply this chemistry.
In order to overcome these major obstacles to using this chemistry, Baran and his group looked to the advancements made in battery manufacturing by teaming up with specialists at the University of Minnesota, headed by Matthew Neurock, PhD, and the University of Utah, headed by Shelley Minteer, PhD.
The lithium-ion, or Li-ion, batteries employed in contemporary electronics like electric cars, laptop computers¸ and mobile phones depend on developments in an internal component referred to as the solid electrolyte interphase, or SEI in short. The SEI is a protective layer forming on one of the electrodes within a Li-ion upon the initial charge of the battery and enables recharging the battery. Creating the safe and efficient batteries currently utilized in consumer electronics depended on decades of developments in improving the chemical conditions, such as the solvents, additives, and composition of electrolytes, which created the SEI.
The researchers observed that the reaction forming the SEI in batteries is an electrochemical reaction similar to the Birch reaction and its lineages. The team eventually concluded that they can possibly borrow from what battery manufacturers had learned to follow a practical and safe way of performing an electroreduction reaction.
In many ways you’re looking at similar situations—powerful reactions that, when effectively harnessed, can provide tremendous utility. The team took advantage of the hard-won knowledge about the conditions that make reductive electrochemistry in batteries practical and used that knowledge to rethink how deeply reductive chemistry could be used on a large scale.
Solomon Reisberg, Study Co-Author, The Scripps Research Institute.
Reisberg is the graduate student in the Baran lab.
The research team at Scripps Research started by testing an array of additives utilized for preventing overcharging in Li-ion batteries and discovered that a combination of two substances, known as TPPA and dimethylurea, helped in achieving the Birch reaction at room temperature.
While testing numerous other materials employed in batteries, Baran’s team developed a range of conditions that enabled them to conduct reductive electrosynthesis in a safe manner and also to increase the reaction’s versatility to produce a broader variation of products that otherwise could not be achieved with earlier electrochemical techniques.
This technique prevented the necessity for dissolving liquid metals in huge amounts of ammonia—and the related risk and cost—and instead, an electrolyte system akin to that utilized in batteries was used. Apart from the Birch reaction, the team was able to use the method on other kinds of intense reactions that are usually employed in synthesis but seldom, if ever, applied in industrial settings.
Chemists have been slow to adopt these electrosynthesis techniques, partially due to concerns about using them safely at scale. This collaboration was able to address existing concerns with an elegant electrochemical solution that could dramatically reduce the costs of synthesizing organic compounds in medicines and other high-value products.
Carol Bessel, PhD, Acting Division Director, Division of Chemistry, National Science Foundation.
Many versions of significant single-ring compounds and also molecules were synthesized by the researchers. Multiple rings in these molecules were integrated to produce more intricate structures that form the framework of chemical products, including drugs. In contrast to the extremely costly devices earlier needed to perform reductive chemistry in huge amounts, the researchers partnered with Asymchem Life Science—a chemical manufacturer based in Tianjin, China—to develop a tiny modular device that has the ability to generate huge amounts of products for below $250.
This demonstrates that kilogram-scale synthesis of pharmaceutically relevant building blocks can be produced by adapting what we’ve learned about electrochemistry from the rapid advance of battery technology. We anticipate that this will be a boon to industry, allowing them to finally bring these reactions to practical use.
Phil Baran, PhD, Study Senior Author and the Darlene Shiley Chair, Department of Chemistry, The Scripps Research Institute.
The Scripps Research researchers partnered on the project with the research team at Pfizer Global Research and Development, the University of Minnesota, the University of Utah, and Asymchem Life Science in China.
Additional authors on the paper, titled "Scalable and safe synthetic organic electroreduction inspired by Li-ion battery chemistry", were Byron K. Peters, Kevin X. Rodriguez, Solomon H. Reisberg, Sebastian B. Beil, David P. Hickey, Yu Kawamata, Michael Collins, Jeremy Starr, Longrui Chen, Sagar Udyavara, Kevin Klunder, Timothy Gorey and Scott L. Anderson.
The study was supported by a National Science Foundation grant for the Center for Synthetic Organic Electrochemistry (CCI Phase 1 grant #1740656), Pfizer, and Asymchem.