Jan 30 2020
Plants absorb solar energy to convert carbon dioxide (CO2) present in the air into sugars and other types of minerals for their metabolic functions and growth.
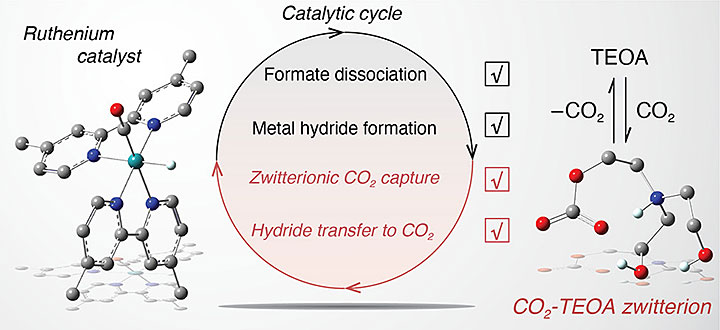 Triethanolamine (TEOA) is actively engaged in key reaction steps of the photocatalytic cycle for the conversion of carbon dioxide (CO2) by ruthenium carbonyl complexes. Image Credit: Journal of the American Chemical Society.
Triethanolamine (TEOA) is actively engaged in key reaction steps of the photocatalytic cycle for the conversion of carbon dioxide (CO2) by ruthenium carbonyl complexes. Image Credit: Journal of the American Chemical Society.
This photochemical reaction can be simulated to efficiently transform atmospheric CO2 into fuels and chemicals, which are important to industries, and ultimately reduce the emission of greenhouse gases and support sustainable energy in the days to come.
To achieve this artificial photosynthesis, researchers have been exploring catalytic systems made up of numerous components. These components work jointly to encourage the transfer of photo-induced electrons needed to change CO2 into products rich in energy.
Formate, a salt form of formic acid, is one such component. Formic acid is an organic chemical that occurs naturally and contains molecules of CO2 and hydrogen. Producing formate from the CO2 gas is believed to be an excellent technique for storing the chemical form of solar renewable energy over a long time.
Generally, multicomponent CO2 conversion systems comprise a catalyst, a photosensitizer, and a sacrificial electron donor in solution. When the photosensitizer absorbs light, it changes to an excited state and begins to accept electrons from the donor.
The function of a catalyst is to reduce the high-energy obstacle to stimulate CO2, which happens to be a highly stable molecule. This catalyst subsequently leverages these high-energy electrons to finish a sequence of reactions.
In many studies focused on the conversion of photochemical CO2 utilizing molecular catalysts such as those based on various metal complexes, including ruthenium, the triethanolamine (TEOA) component is the one that donates the electrons. Alternatively, in certain cases, TEOA ensures its sacrificial behavior by accepting a proton—a positively-charged hydrogen ion—from an electron donor that is more efficient.
In spite of the extensive use of TEOA, most of the studies performed so far have not explored the possibility of the secondary roles played by this component, for example, boosting the rate of reactions or altering the fleeting chemical species (intermediates) produced inside the catalytic cycle.
Now, a group of chemists from the City University of New York’s Baruch College and the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory has set out to change all that.
For the last 40 years, most studies on CO2 reduction catalysts have focused on analyzing catalytic efficiency and selectivity for the final product. However, it is important to know if and how TEOA interacts with the catalyst during intermediate steps of the catalytic cycle because these interactions may decisively influence the efficiency and selectivity of product formation.
Renato Sampaio, Research Associate, Artificial Photosynthesis Group, Chemistry Division, Brookhaven National Laboratory
The chemists made some unexpected findings by targeting a popular catalytic system in an acetonitrile solution containing a common electron donor called BIH, a ruthenium-based photosensitizer, a ruthenium carbonyl (carbon atom coupled to an oxygen atom) catalyst, and TEOA behaving as a proton acceptor to encourage the BIH’s sacrificial behavior.
They demonstrated that TEOA failed at its main intended task and did not efficiently accept protons from BIH, thereby restricting the activity of catalysts. But it was also discovered that TEOA improves the crucial steps of the catalytic cycle to change CO2 into the target product, that is, formate. The study was reported in a paper published online in the Journal of the American Chemical Society, on December 27th, 2019.
For instance, TEOA behaves as a source of proton supporting the creation of a metal hydride (ruthenium bound to hydrogen) that further communicates with the CO2 gas to form bound formate, that is, attached to ruthenium.
TEOA also forms a “zwitterionic adduct” by interacting with CO2. The zwitterionic adduct is a molecule comprising both negative and positive electrical charges. When a solution contains this adduct, interaction occurs between the CO2 and metal hydride to create bound formate at six orders of magnitude quicker than that without TEOA.
Moreover, the dissociation of this bound formate into “free” formate is also six orders of magnitude quicker because of the TEOA component. The “free” formate can be captured as the end product. The researchers made these determinations by collecting both spectroscopic and electrochemical data.
The catalytic cycle can generate a large number of reaction intermediates. The challenge is characterizing them by spectroscopic or electrochemical techniques.
Etsuko Fujita, Study Co-Corresponding Author and Group Leader, Artificial Photosynthesis Group, Chemistry Division, Brookhaven National Laboratory
The researchers initially quantified the catalyst’s reduction potentials (that is, how effortlessly the catalyst gains an electron) both in the absence and presence of the TEOA component. They subsequently defined the carbonyl’s spectroscopic vibrations for varying forms of the catalyst, both before and after it gains an electron.
After making these electrochemistry measurements, the scientists tracked the catalytic intermediates in the atmospheric CO2 by performing time-resolved infrared spectroscopy experiments on nanosecond timescales.
The carbonyl that is bound to ruthenium enabled us to study each transient intermediate form of the catalyst. The carbonyl is a very sensitive infrared spectroscopic reporter that remains bound to ruthenium throughout the catalytic cycle, unlike other parts of the catalyst.
Renato Sampaio, Research Associate, Artificial Photosynthesis Group, Chemistry Division, Brookhaven National Laboratory
Sampaio continued, “Its vibrational frequency, or atomic motion, dramatically shifts as the catalyst accepts an electron or undergoes other structural changes. We can detect these shifts and view them alongside the electrochemistry measurements to tell which species are present.”
In upcoming research, the scientists will analyze TEOA alternatives that not only increase the sacrificial potential of the BIH but also provide analogous benefits in improving the catalytic cycle.
“Even though our study focused on a specific class of catalysts, we strongly believe that our findings are broadly applicable across other systems and should be taken into consideration when investigating the catalytic reduction of CO2 to formate,” concluded Fujita.