Feb 26 2020
A team of researchers from the Tokyo Institute of Technology (Tokyo Tech) and ERATO Japan Science and Technology has demonstrated a new method to synthesize superatoms of a preferred volume, stability, and valency in a solution medium by modifying the number of atoms present in a cluster structure.
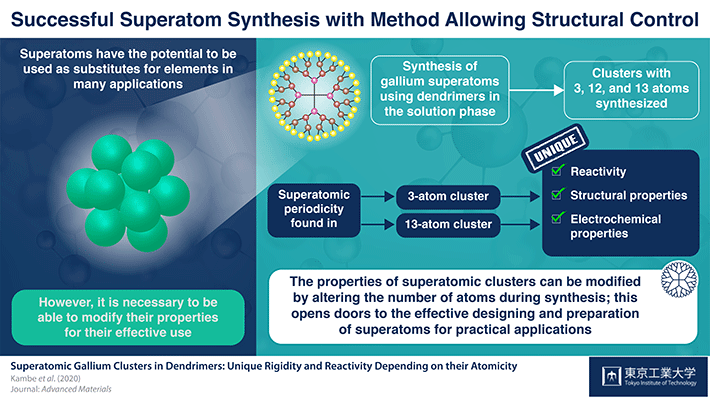 Schematic illustration of superatomic clusters with 3, 12, and 13 atoms. Image Credit: Tokyo Institute of Technology.
Schematic illustration of superatomic clusters with 3, 12, and 13 atoms. Image Credit: Tokyo Institute of Technology.
This development represents a major step in achieving the viable application of the clusters of superatoms as alternatives to elements used in chemical reactions.
'Superatom' is a term given to a group of atoms that appears to display similar properties as elemental atoms. Researchers have shown a specific interest in the structures of superatoms because these structures can be coupled with atoms to create molecules and can possibly be utilized to replace specific elements in several applications.
However, to use the superatoms effectively, they should be uniquely customized to look like the properties of the matching elements. Such a transformation relies on the particular combination of the electrons utilized.
For instance, when an aluminum atom containing three valence electrons (outer shell electrons that play a role in the chemical bond formation) is introduced in the superatom of aluminum-13, the properties become similar to those of a superatom of aluminum-14.
Owing to the modifiable nature of superatoms, it is important to further study and interpret these atoms. However, earlier research has been mostly conceptual and mainly focused on single clusters of atoms. In addition, the study was unable to produce superatomic clusters that have adequate stability and volume for viable applications.
In a new analysis, scientists from ERATO Japan Science and Technology and Tokyo Tech created clusters of the gallium (Ga) element in a solution to show that altering the number of atoms in a cluster has an impact on the cluster properties. The study was headed by Dr. Tetsuya Kambe and Professor Kimihisa Yamamoto.
With the help of a specialized superatom synthesizer, the researchers produced Ga clusters of 3, 12, 13, as well as other numbers of atoms.
To define and investigate the structural variations that occur among the produced clusters, transmission electron microscopic images were recorded and computation tools were used to perform the calculations.
The mass spectrometry showed that the clusters of 13- and 3-atoms had superatomic periodicity. The cluster of 13-atoms varied from the other clusters electrochemically and structurally. However, the 3-atom cluster with hydrogen (Ga3H2) was converted to Ga3H2– and this was not identified. This indicates the low stability of this cluster when produced in the solution medium.
The potential to modify the clusters strengthens the concept that superatoms can be induced with structural modification. The scientists thus elucidated the implications of their findings.
These series of results demonstrate that it is possible to change the valence electrons in superatomic clusters in solution by controlling the number of constituent atoms. This, in turn, enables the designing and preparation of superatoms.
Scientists, Tokyo Institute of Technology and ERATO Japan Science and Technology
The research presents new opportunities for upcoming studies to analyze the application of superatoms as alternatives to elements. As Dr. Kambe, Professor Yamamoto, and the researchers reiterate, “the superatom reveals an attractive strategy for creating new building blocks through the use of cluster structures.”