Mar 3 2020
When a solid material is sufficiently heated, the thermal energy, or latent heat, makes the molecules of the material break apart and form a liquid. Ice turning into water is one of the popular examples of this phase transition from a well-ordered solid-state to a less-ordered liquid state.
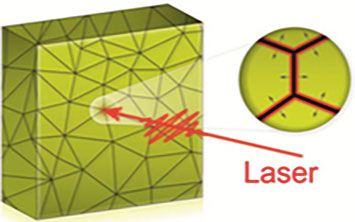 An illustration of grain boundary locations (points where lines intersect) in a polycrystalline gold thin film. The zoomed-in view shows how a melt front created at these boundaries propagates into the grains after the film is excited with an optical laser. Image Credit: Brookhaven National Laboratory.
An illustration of grain boundary locations (points where lines intersect) in a polycrystalline gold thin film. The zoomed-in view shows how a melt front created at these boundaries propagates into the grains after the film is excited with an optical laser. Image Credit: Brookhaven National Laboratory.
Although melting is known to be an underlying process of matter, researchers are yet to completely understand the way it functions at a microscopic level due to the lack of research capabilities with adequate time resolution. But the introduction of X-ray free-electron lasers (XFELs) in the past 10 years has enabled the study of the melting mechanism as well as other kinds of ultrafast atomic-scale dynamics.
Such instruments utilize free, or unbound, electrons to produce femtosecond (one-quadrillionth of a second) pulses of light in the X-ray energy region. When compared to X-ray synchrotrons, XFELs have X-ray pulses of higher intensity and a relatively shorter duration.
A group of international researchers has now utilized one of these instruments known as the Pohang Accelerator Laboratory XFEL (PAL-XFEL) in South Korea to track the melting of nanometer-thick gold films composed of many tiny crystals oriented in numerous directions.
The researchers used an ultrashort X-ray pulse (probe) to track the structural variations after these polycrystalline gold thin films were excited by a femtosecond laser (“pump”). This femtosecond laser includes melting. As soon as the X-ray pulse hits the gold, the X-ray beam becomes diffracted in a pattern that is typical of the crystal structure of the material.
When the researchers gathered the X-ray diffraction images at varying pump-probe time delays on picosecond, or one-trillionth of a second, scales, they took “snapshots” as melting started and continued in the gold thin films.
Variations in the diffraction patterns over time showed the dynamics of crystal disordering. For this analysis, the researchers chose gold because it has a well-defined solid-to-liquid transition and diffracts X-rays quite powerfully.
The X-ray diffraction patterns also showed that melting is non-uniform, or inhomogeneous. In a study that appeared online in the January 17th, 2020, issue of Science Advances, researchers suggested that this melting could have originated at the interfaces in which crystals of varying orientations meet (imperfections known as grain boundaries) and subsequently propagate into the tiny crystalline region (grains). To put this in simpler terms, the grain boundaries begin to melt before the rest of the crystal.
Scientists believed that melting in polycrystalline materials occurs preferentially at surfaces and interfaces, but before XFEL the progression of melting as a function of time was unknown. It was known that the laser generates ‘hot’ (energetic) electrons, which cause melting when they transfer their energy to the crystal.
Ian Robinson, Study Co-Corresponding Author, Brookhaven National Laboratory
Robinson continued, “The idea that this energy transfer process happens preferentially at grain boundaries and thus is not uniform has never been proposed until now.”
Robinson is also the leader of the X-ray Scattering Group in the Condensed Matter Physics and Materials Science (CMPMS) Division at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory.
The mechanism of laser-induced melting is important to consider for micromachining of precision parts used in aerospace, automotive, and other industries.
Tadesse Assefa, Study First Author and Postdoc, Brookhaven National Laboratory
Assefa, who is a postdoc in Robinson’s group, continued, “The way the laser couples to the material is different depending on the pulse duration of the laser. For example, the ultrashort pulses of femtosecond lasers seem to be better than the longer pulses of nanosecond lasers for making clean cuts such as drilling holes.”
For their experiment, the researchers initially developed thin films of differing thicknesses (50, 100, and 300 nm) at the Center for Functional Nanomaterials (CFN), a DOE Office of Science User Facility at Brookhaven National Laboratory.
At the CFN Nanofabrication Facility, the researchers carried out electron-beam evaporation, a kind of deposition method that utilizes electrons to condense the required material onto a substrate. The facility’s ultraclean atmosphere allowed the team to make gold films of even thickness across a large sample area.
At PAL-XFEL, the research team carried out time-resolved X-ray diffraction on gold films across an array of laser power levels. Staff in Brookhaven Laboratory’s Computational Science Initiative developed software that managed the high-throughput analysis of the terabytes of data produced as a detector gathered the diffraction pattern images.
Then, using software created by colleagues at Columbia Engineering, the researchers transformed these images into linear graphs. The plots in the graphs showed a double peak that corresponded to a “hot” region undergoing melting (intermediate peak) as well as a comparatively “cold” region (the rest of the crystal) which is yet to get the latent heat of melting.
Via the electron coupling process, heat reaches up to the grain boundaries and subsequently conducts into the grains. Such an uptake of latent heat leads to a band of melting material closely packed between a pair of moving melt fronts. Eventually, this band becomes larger.
“One melt front is between a solid and melting region, and the other between a melting and liquid region,” stated Robinson.
The researchers have now planned to verify their two-front model by decreasing the grain’s size (thus increasing the amount of grain boundaries) so that they can approach the end of the melting process. Because melting takes place as a wave traversing the crystal grains at a comparatively slow speed at (30 m/second), it takes more time when compared to the timing range of the instrument (500 ps) to cross large grains.
The researchers would also like to explore other kinds of alloys (mixtures of numerous metals or a metal integrated with other elements), metals, as well as catalytically relevant materials in which grain boundaries take part in chemical reactions.
This study represents the very beginning of how we build an understanding of the mechanism of melting. By performing these experiments using different materials, we will be able to determine if our model is generalizable.
Tadesse Assefa, Study First Author and Postdoc, Brookhaven National Laboratory
Pohang University of Science and Technology, University College London, and Sogang University are other co-operating institutions.