Mar 6 2020
Researchers have successfully created an active sulfur material and carbon nanofiber (CNF) composite using a simple and low-cost liquid phase process.
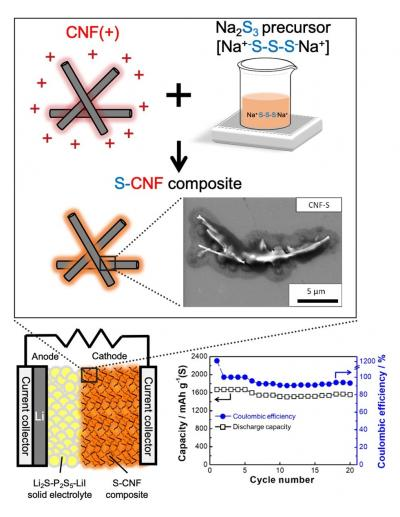 Schematic images and electron microscope photograph of sulfur-carbon composites (upper). Schematic images and cycle characteristics of all-solid-state sulfur battery (lower). Image Credit: Toyohashi University of Technology.
Schematic images and electron microscope photograph of sulfur-carbon composites (upper). Schematic images and cycle characteristics of all-solid-state sulfur battery (lower). Image Credit: Toyohashi University of Technology.
The research team included Professor Atsunori Matsuda, Professor Hiroyuki Muto, Assistant Professor Kazuhiro Hikima, Assistant Professor Nguyen Huu Huy Phuc, Researcher Reiko Matsuda, and Mr Takaki Maeda (Master Program) from the Department of Electrical and Electronic Information Engineering at Toyohashi University of Technology.
When compared to lithium-ion secondary batteries, all-solid-state lithium-sulfur batteries based on a sulfur-CNF composite material— produced by the low-cost, simple liquid-phase process—show a more improved cycle stability and higher discharge capacity. Hence, all-solid-state lithium-sulfur batteries could be used in large-scale batteries, like electric vehicles, in the days to come.
Details
Last year, the Nobel Prize in chemistry went to lithium-ion secondary batteries, which have been extensively utilized as power sources for electric vehicles, smartphones, and so on.
In recent years, all-solid-state lithium batteries gained traction as state-of-the-art batteries and this trend can be attributed to the rise in electric and hybrid vehicles. These batteries specifically gained attention because their energy density is five times higher than that of traditional lithium-ion secondary batteries.
But sulfur is an insulator, which therefore restricts their use in battery devices. Hence, to overcome this problem, sulfur should be provided with an ionic and electron -conductive path.
The researchers proposed that cathode composites obtained by integrating a carbon nanofiber (CNF) and sulfur active material using an electrostatic assembly technique. This method can evenly integrate materials into a solution.
All-solid-state lithium-sulfur batteries, using electrochemically stable Li2S-P2S5-LiI solid electrolytes and sulfur-CNF composites produced by the liquid-phase process, displayed high discharge capacity that was equal to sulfur’s theoretical capacity and sustained high capacity following continuous charge-discharge cycles.
Nguyen Huu Huy Phuc, the study’s first author and Assistant Professor from the Toyohashi University of Technology, described the features of the all-solid-state lithium-sulfur battery.
It is required that a sulfur active material and a carbon material are appropriately combined for making high-performance all-solid-state lithium-sulfur batteries. Conventionally, sulfur-carbon composites were synthesized by mechanical mixing, liquid mixing using a special organic solvent and complicated methods, in which sulfur is combined with a porous carbon material with a high specific surface area.
Nguyen Huu Huy Phuc, Study First Author and Assistant Professor, Toyohashi University of Technology
Nguyen Huu Huy Phuc continued, “However, there were few reports that all-solid-state lithium-sulfur batteries showed high capacity almost equivalent to the theoretical capacity of sulfur and high cycle stability. Therefore, we focused on making a sulfur-carbon composite using a low-cost and simple electrostatic adsorption method which can uniformly combine nanomaterials.”
It was confirmed that sulfur at the sulfur-carbon composite synthesized by electrostatic adsorption method was accumulated on carbon nanofiber in the form of sheets. Besides, we constructed all-solid-state lithium-sulfur batteries and found that sulfur was fully utilized as an active material. The other merit is that this sulfur-carbon composite can be produced by lower cost than conventional processes.
Nguyen Huu Huy Phuc, Study First Author and Assistant Professor, Toyohashi University of Technology
Development Background
In the electrostatic adsorption technique, smaller particles and larger mother particles are electrostatically adsorbed by altering the particles’ surface charges using polyelectrolytes to promote an electrostatic interaction.
Although many different ceramic composites have already been designed using the electrostatic adsorption technique, it was difficult to adjust the sulfur’s surface charges.
But the researchers were able to make the charge adjustment by utilizing chemical reactions, where S and Na2S formed aqueous soluble Na2S3 by reacting in ion-exchanged water. Hence, this research was able to achieve a novel chemical process by using the basic principle of electrostatic adsorption.
Future Outlook
The liquid phase process is a relatively simple and low-cost technique for preparing composites of carbon and sulfur, thus making it appropriate for large-scale production. Through this technique, all-solid-state lithium-sulfur batteries utilizing a sulfur active material can be put to practical use.
Furthermore, the energy density of electric vehicles, as well as large-sized power source batteries for business and household use, is expected to improve exponentially.
The research was funded by the Advanced Low Carbon Technology Specially Promoted Research for Innovative Next-Generation Batteries (ALCA-SPRING, JPMJAL1301) program of the Japan Science and Technology Agency (JST).