Jan 12 2021
Natural gas produces comparatively lower amounts of pollutants such as carbon dioxide—a powerful greenhouse gas and the main contributor to climate change—and thus, it is the cleanest conventional fossil fuel source.
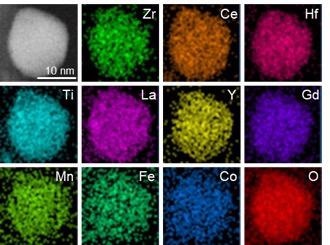 The elements that make up the metal oxide nanoparticle catalyst. Image Credit: Zhennan Huang.
The elements that make up the metal oxide nanoparticle catalyst. Image Credit: Zhennan Huang.
However, pollutants and greenhouse gases discharged by the combustion of natural gas could be decreased much more by using sophisticated catalysts that help reduce the temperature at which methane—the main component of natural gas—is burned.
The more efficiently we burn methane and the less energy we use to burn it, the less greenhouse gas and pollutants it produces. So, anything we can do to get this combustion temperature down is an environmental win.
Reza Shahbazian-Yassar, Professor of Mechanical and Industrial Engineering, University of Illinois Chicago
Shahbazian-Yassar and his team enabled formulating an advanced “Swiss Army knife” catalyst formed of 10 different elements—where each element has its own ability to decrease the temperature at which methane burns—as well as oxygen. This exclusive catalyst can reduce methane’s combustion temperature by nearly half—from more than 1400 K down to 600–700 K.
The results of the study have been published in the Nature Catalysis journal.
In a study published earlier, Shahbazian-Yassar and his team showed the ability to develop multi-element nanoparticle catalysts, called high-entropy alloys, with the help of a special shock-wave technique. Prior to that, no serious efforts were made by materials scientists to develop nanoparticles using more than three elements due to the tendency of each elements’ atoms to get isolated from each other and turn useless.
Shahbazian-Yassar’s team leveraged the exclusive real-time, high-temperature electron microscopy system at UIC to demonstrate that high-entropy nanoparticles composed of 10 metal oxides were extremely stable at temperatures of up to 1,073 K and the individual elements were evenly distributed throughout each nanoparticle, thereby forming a single, solid-state stable crystalline structure.
The newly developed metal oxide alloy included different blends of transition metals—which are rare-earth elements—and noble metals, as well as oxygen.
It is almost impossible to maintain a perfect mix of these elements in a solid phase due to the differences in atomic radius, crystal structure, oxidation potential, and electronic properties of the elements. But we were able to show that this is possible.
Zhennan Huang, Study Co-First Author, University of Illinois Chicago
Huang is a PhD student in Shahbazian-Yassar’s lab.
Among multiple alloys with multiple elements that we created, the particles made of 10 elements not only were most effective in reducing the combustion point of methane gas but also the most stable at those temperatures.
Reza Shahbazian-Yassar, Professor of Mechanical and Industrial Engineering, University of Illinois Chicago
Shahbazian-Yassar is the corresponding author of the study.
The team is confident that the catalyst can be applied to decrease the output of hazardous greenhouse gases synthesized while burning natural gas in individual households, to drive turbines, and even in cars running on compressed natural gas.
Co-authors of the study include Tangyuan Li, Yonggang Yao, Menghao Yang, Jinglong Gao, Alexandra Brozena, Liangbing Hu, Yifei Mo, Glenn Pastel, Miaolun Jiao, Qi Dong, Jiaqi Dai, and Shuke Li from the University of Maryland; Pengfei Xie, Kaizhu Zeng, Han Zong, and Chao Wang from Johns Hopkins University; Zhenyu Liu and Guofeng Wang from the University of Pittsburgh; Miaofang Chi from Oak Ridge National Laboratory; and Jian Luo from the University of California, San Diego.
Journal Reference:
Li, T., et al. (2021) Denary oxide nanoparticles as highly stable catalysts for methane combustion. Nature Catalysis. doi.org/10.1038/s41929-020-00554-1.