Mar 8 2021
Individuals who have owned a smartphone for more than a year likely know that the integrated lithium (Li)-ion battery does not retain as much charge as it did when the phone was new.
 The BP copolymer offers several advantages that put it miles ahead of the conventional PVDF binder in terms of stability and durability. Image Credit: Noriyoshi Matsumi from the Japan Advanced Institute of Science and Technology.
The BP copolymer offers several advantages that put it miles ahead of the conventional PVDF binder in terms of stability and durability. Image Credit: Noriyoshi Matsumi from the Japan Advanced Institute of Science and Technology.
The degradation of such Li-ion batteries is a major concern that considerably reduces the useful life of handheld electronic devices, indirectly resulting in large amounts of economic losses and pollution.
As well as these problems, the fact that Li-ion batteries are not highly long-lasting is a huge barrier for the renewable energy harvesting and electric vehicle market.
Given the extent of these problems, it is no wonder that scientists have been intensely looking for means to enhance the advanced designs of Li-ion batteries.
One of the crucial causes for the reduced capacity of Li-ion batteries over time is the degradation of the extensively utilized graphite anodes—that is, the negative terminals found in batteries.
The anode, along with the electrolyte (or the medium carrying the charge between two terminals) and the cathode (or the positive terminal), offers an environment in which the electrochemical reactions for the charge-discharge cycle of the battery can occur.
But graphite needs a binder to prevent it from disintegration with use. Poly(vinylidene fluoride) (PVDF)—the most extensively used binder today—has several disadvantages that make it far from a perfect material.
To deal with these problems, a research team from the Japan Advanced Institute of Science and Technology (JAIST) is analyzing a new kind of binder produced from a bis-imino-acenaphthenequinone-paraphenylene (BP) copolymer.
Published in the ACS Applied Energy Materials journal, the new study was headed by Professor Noriyoshi Matsumi and also included Professor Tatsuo Kaneko, Senior Lecturer Rajashekar Badam, PhD student Agman Gupta, and former postdoctoral fellow Aniruddha Nag.
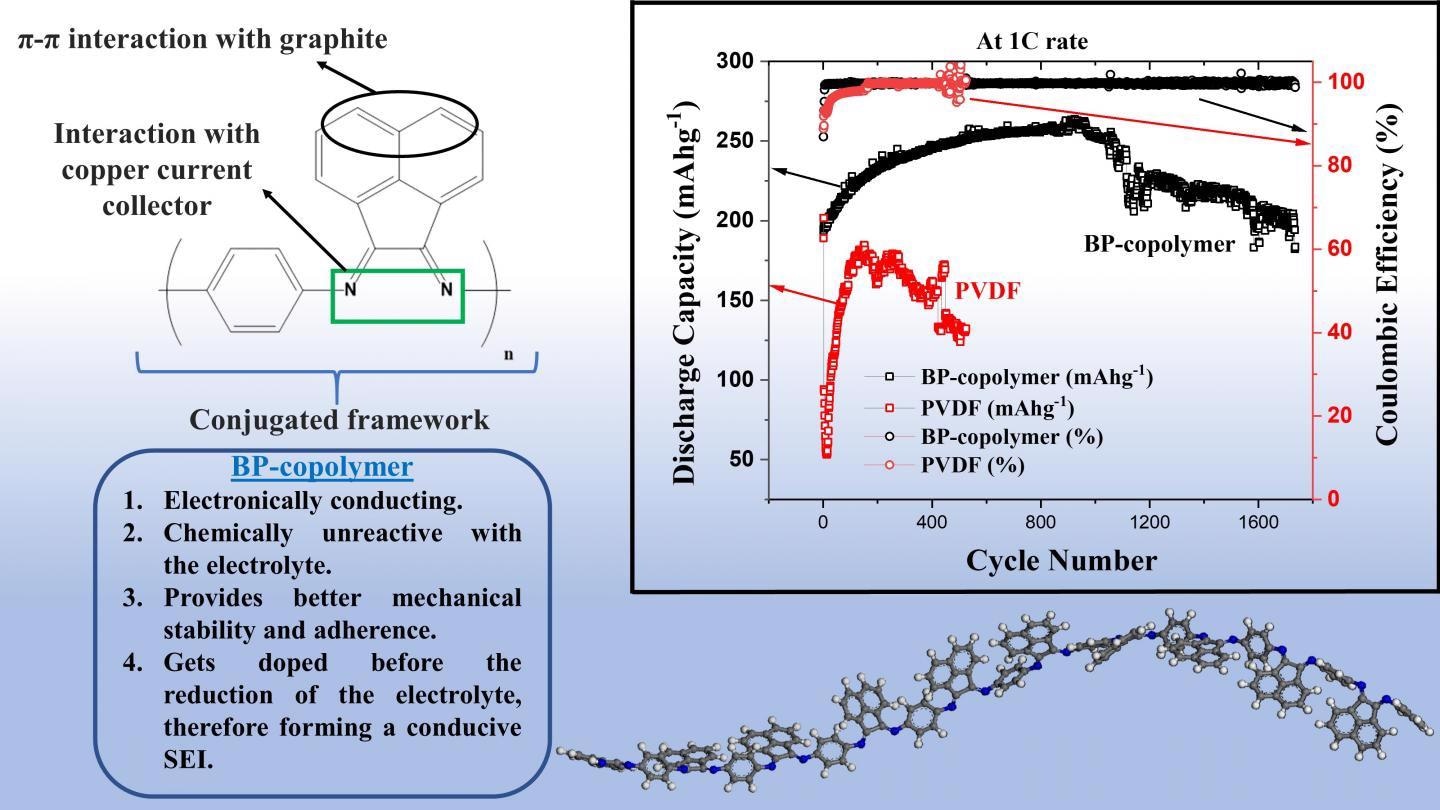
So, in what respect does the BP copolymer outpace the traditional PVDF binder for graphite anodes? First and foremost, the BP binder provides considerably better adherence and mechanical stability to the anode.
This partly comes from the supposed π-π interactions between graphite and the bis-imino-acenaphthenequinone groups as well as from the excellent adherence between the ligands of the copolymer and the copper current collector of the battery.
Secondly, the BP copolymer is relatively more conductive than PVD and it also creates a thinner conductive solid electrolyte interface that has less resistance. And thirdly, the BP copolymer does not easily react with the electrolyte, which also considerably avoids its degradation.
The combination of these benefits resulted in some serious performance enhancements, as the investigators showed through experimental measurements.
Whereas a half-cell using PVDF as a binder exhibited only 65% of its original capacity after about 500 charge-discharge cycles, the half-cell using the BP copolymer as a binder showed a capacity retention of 95% after over 1700 such cycles.
Noriyoshi Matsumi. Professor, Japan Advanced Institute of Science and Technology
The half-cells based on the BP copolymer demonstrated an extremely high and stable coulombic efficiency—a measure that compares the extent of charge flowing in and out of the cell in a specified cycle; this also indicates the enduring durability of the battery.
Using a scanning electron microscope, pictures of the binders were taken both before and after cycling and these images showed that only small cracks had developed on the BP copolymer, while the PVDF already contained large cracks in less than a third of the overall number of cycles.
Both the experimental and theoretical results of this research work will present new avenues for designing durable Li-ion batteries.
Consequently, this may have far-reaching environmental and economic consequences, as explained by Professor Matsumi, “The realization of durable batteries will help in the development of more reliable products for long-term use. This will encourage consumers to purchase more expensive battery-based assets like electric vehicles, which will be used for many years.”
Professor Matsumi also added that long-lasting batteries would be good news for those depending on artificial organs, like patients suffering from certain heart problems. Evidently, the general population would also gain much, considering the number of laptops, tablets, and smartphones being used and recharged daily.
Additional developments in electrode binders are expected to bring scientists closer to more long-lasting battery-based products and, thus, a greener future.
Journal Reference:
Gupta, A., et al. (2021) Bis-imino-acenaphthenequinone-Paraphenylene-Type Condensation Copolymer Binder for Ultralong Cyclable Lithium-Ion Rechargeable Batteries. ACS Applied Energy Materials. doi.org/10.1021/acsaem.0c02742.