May 31 2021
Protons are subatomic particles with a positive electric charge and are one of the first particles to have developed following the evolution of the universe. They are also a constituent of each atom existing now.
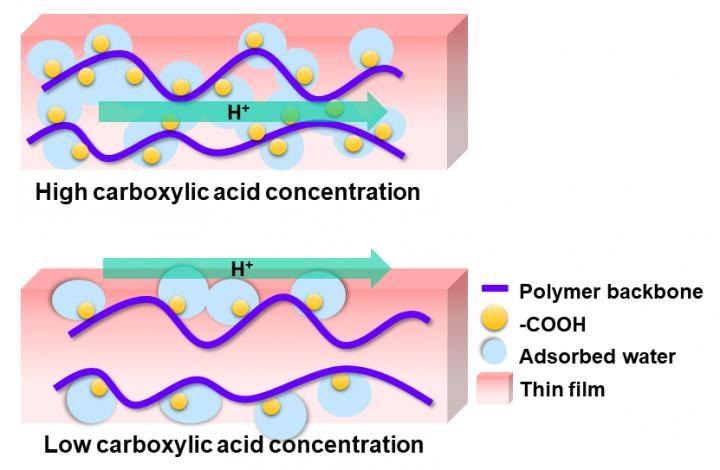
Schematic representation of the dependence of the main proton transport pathway in polymer thin films on the carboxylic acid group concentration. Image Credit: Yuki Nagao.
In biological systems. proton movement tends to play a major role in the processes of energy conversion, like respiration and photosynthesis. Besides, proton conduction is a key factor for hydrogen fuel cells, which have often been solicited as the perfect clean energy source for the next generation.
High proton conduction that has been noted in biomaterials like protein and sugar derivatives is due to the existence of proton-donating functional groups (substituents present in a molecule that regulates its characteristic chemical reactions).
But the accurate mechanism of proton transport in such materials has not been understood clearly until now (for example, if the protons choose to flow through the biomaterial surface, that is, interfacial transport, or via the bulk). Furthermore, it is uncertain how the concentration of the functional group may impact the proton transport pathway.
Against this background, in a new study reported recently in the journal Electrochemistry, a research group from Japan, headed by Associate Professor Yuki Nagao from Japan Advanced Institute of Science and Technology (JAIST) and Athchaya Suwansoontorn, a PhD student at JAIST, as well as Associate Professor Katsuhiro Yamamoto from Nagoya Institute of Technology, Professor Shusaku Nagano from Rikkyo University and Professor Jun Matsui from Yamagata University, set out to examine how proton conduction was impacted in styrene-based polymers with the alteration in the concentration of carboxylic acid, a proton-donating organic acid found generally in biomaterials.
Suwansoontorn laid out the encouragement for their study titled, “The study of proton transport pathways is fundamentally important to elucidate the functioning of many biological systems.”
The research group systematically produced polymers with various concentrations of carboxylic acid and made them as thin films with a high surface-to-bulk ratio to allow examination of interfacial transport properties. After this, they characterized the polymer structures using a range of standard characterization methods.
The existence of two types of carboxylic acid groups (COOH) in the polymers was noted by the research team. One was free COOH groups, which were more plentiful at higher concentrations, and the other one was cyclic dimer COOH groups, which were widespread at low concentrations.
To synchronize this with proton transport, the scientists investigated in-plane proton conduction with the help of impedance spectroscopy and estimated the interfacial resistance to measure the potential of interfacial transport.
They discovered that a high COOH concentration was greatly conducive to internal proton transport while lower concentrations were conducive to interfacial transport. They credited this to the existence of free COOH groups at high concentrations that produced more hydrogen bonding networks, thereby making proton conduction easier.
Moreover, they confirmed this concept by demonstrating that a greater number of free COOH groups on the interface resulted in higher interfacial conduction.
Our research may contribute to developing bio-conductive materials for biological devices involved with proton conduction and eco-friendly fuel cells. From a broader perspective, it can facilitate people's lives by supporting biological technologies and green energy application development.
Athchaya Suwansoontorn, PhD Student, Japan Advanced Institute Of Science and Technology
Suwansoontorn considered the practical ramifications of the results. To mitigate climate change, greener energy is an emergent need. In that regard, the study results guarantee certain intriguing impacts to look forward to, for sure!
Journal Reference:
Suwansoontorn, A., et al. (2021) Interfacial and Internal Proton Conduction of Weak-acid Functionalized Styrene-based Copolymer with Various Carboxylic Acid Concentrations. Electrochemistry. doi.org/10.5796/electrochemistry.21-00042.