Pollution because of toxic heavy metals in water and soil poses a serious threat to human health. Despite the fact that there has been a lot of work done on reporting heavy metal pollutions around the world, there are few review articles on how to deal with this problem. Research in the Royal Society Open Science Journal examines inorganic nanoparticles and lays out the criteria for selecting them as suitable nanoadsorbents for removing heavy metals from water.

Study: Heavy metal pollution and the role of inorganic nanomaterials in environmental remediation. Image Credit: Silent Corners/Shutterstock.com
Various inorganic nanoparticles, such as metal, metal oxide, and metal sulfide nanoparticles, have indeed been successfully used as nanoadsorbents to remediate water with high heavy metal pollution.
Pollutants as a Global Issue
Water and soil pollution is a global issue. Toxic metals including mercury (Hg), lead (Pb), cadmium (Cd), and arsenic (As) are extremely poisonous, posing a major hazard to human and other domestic animals’ health at even low quantities in water and soil.
Mercury has harmful effects on the human kidney, brain, and nervous system, whilst lead contamination has been linked to neurodevelopmental problems, and cadmium is a carcinogen [1,2]. Dermal blemishes, skin malignancies, and bladder and lung cancers are all caused by arsenic consumption.
The development of illegal gold panning and mining activities using mercury-gold amalgamation technologies has been described as a major source of mercury contamination in most developing nations. Lead and cadmium are commonly released into the environment through the manufacturing of batteries, metals, plastics, and fertilizers, as well as mining. In subsurface fluids with sulfide and sediment layers, arsenic pollution is common.
Common Pollutant
The most common types of lead in the environment are inorganic lead (Pb2+) compounds. Organic lead compounds that were once connected using leaded petrol and paints have indeed been phased out. A significant amount of research on the amounts of heavy metal contamination in the environment has been widely documented, laying the groundwork for creating remediation sequestration solutions.
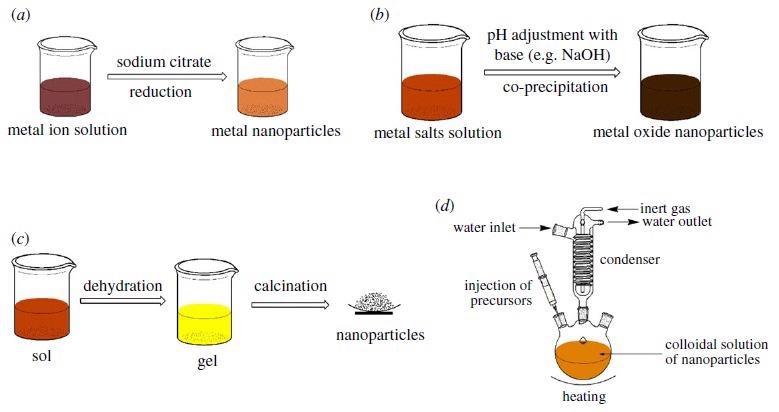
Synthesis pathways for nanoadsorbents: (a) reduction, (b) co-precipitation, (c) sol–gel and (d) hot-injection methods. Image Credit: Mensah, M., et al, Royal Society Publications
Activated carbon, zeolite, silica gel, activated alumina, natural clay (kaolinite, bentonite, illite, stevensite, and rectorite), and biomass are the most commonly utilized adsorbents for heavy metals sequestration.
Adsorption Products for Contamination
These adsorption products are suitable. However, they lack selection and have a low affinity for the heavy metals that are being targeted. Adsorbents can be created to have improved qualities compared to ordinary bulk material adsorbents with the advent of nanoscience, which incorporates methods for scaling down material dimensions to the nanoscale (1–100 nm).
Nanoscale adsorbents have enhanced selectivity, capacity, and affinity for heavy metal contaminants, as well as providing faster and more effective adsorption processes.
Nanoscale adsorbents also have a high surface area to volume ratio, surface structural features designed for a specific application, a short diffusion path or no internal diffusion resistance, high porosity, improved structural characteristics, and catalytic capabilities.
Nanomaterials as an Adsorbents
Nanomaterials are nanoscale substances with better performance than bulk materials, which are referred to as emergent characteristics. Because of the size effect caused by quantum confinement, nanoscale materials can be exploited as effective adsorbents in environmental cleanup operations.
Inorganic, carbon-based, polymer, nanocomposite, and biomaterials are all examples of nanomaterials. Inorganic nanoparticles have been widely used in environmental cleanup.
Iron oxides are the most commonly utilized magnetic metal oxides. Because they can be swiftly separated from the solution using an external magnet, they have a wide range of applications as nano adsorbents. Metal sulfides have a higher affinity for heavy metal ions than metal oxides, but they have received less attention as nanoadsorbents for the removal of heavy metals from water.
The contaminants chemisorb on the nanoadsorbent's surface, implying that chemical linkages are created between the nanoadsorbent and the pollutant. The desorption process of a good nanoadsorbent should be simple. The most desirable adsorbents are those that have a high recycling or regeneration potential.
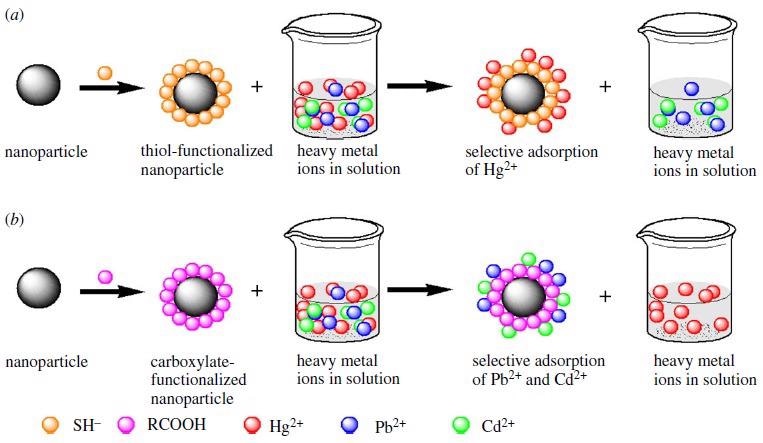
Selective adsorption of heavy metal ions in a solution based on the Pearson’s theory of hard and soft acids and bases using inorganic nanoparticle (a) thiol functionalized nanoparticle selectively adsorbs Hg2+ and (b) carboxylate functionalized nanoparticle selectively adsorbs Pb2+ and Cd2+. Image Credit: Mensah, M., et al, Royal Society Publications
It has been proven that inorganic nanoadsorbent materials can help to clean up the environment. Over 100 mg l1 of heavy metal pollution has been eliminated. Certain nanoadsorbents have the competence to implement other roles besides adsorption, such as amalgamation. Gold may amalgamate with mercury, but it must be in the reduced state Hg0 for this to happen. Not all nanoadsorbents are capable of forming stable amalgams with the pollutant of interest. In most cases, mercury contamination in water is primarily caused by Hg2+ ions.
Metal nanoparticles, such as copper, silver, tin, and aluminum that form a stable amalgam with mercurial are appealing, and potential nanoadsorbents for mercury contamination sequestration. Although iron is an excellent magnetic absorbent, it does not create a stable amalgam with mercury. Nevertheless, by doping nanoparticles like nZVI with metals that amalgamate with mercury, a more permanent amalgam may be formed, ensuring the successful development of a more effective and permanent nanoadsorbent for Hg2+ contaminant removal from water.
References
Mensah, M., et al. Heavy metal pollution and the role of inorganic nanomaterials in environmental remediation. Royal Society Publications. Published:13 October 2021 https://royalsocietypublishing.org/doi/10.1098/rsos.201485
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.