A new study in the journal Energy aims to consolidate critical data on the CO2 capture capacity of adsorbents generated from waste plastic.

Study: Transforming Plastic Waste into Porous Carbon for Capturing Carbon Dioxide: A Review. Image Credit: Teerasak Ladnongkhun/Shutterstock.com
As an adsorbent, activated carbon plastic waste might be a potential way to concurrently address two environmental challenges: CO2 emissions and solid waste disposal. Furthermore, the future prospects for char made from waste plastics are discussed.
Plastic Waste Haphazard
Each day, the amount of plastic waste produced increases dramatically; every year, the amount of plastic waste produced increases to a cumulative estimate of 300 billion kilograms. Most plastic materials would be broken down into smaller particles rather than being destroyed or disappearing
The haphazard disposal of plastic waste can have a number of negative environmental consequences, including an increase in greenhouse gases and water pollution. As a result, it is prudent to consider other options for reducing plastic waste without negatively impacting the environment, such as transforming it into valuable products using efficient strategies such as pyrolysis.
Products of the pyrolysis process, which include liquid, gas, and solid residues, can be converted into useful products, with the liquid product serving as a transportation fuel and char serving as an excellent sorbent. Plastic waste char could be modified to improve carbon dioxide (CO2) adsorption effectiveness.
Conventional Ways
The conventional method of reducing the amount of plastic waste has involved landfilling, in which plastics are ditched at a particular land site. This is an ineffective method of reducing plastic waste, which causes serious environmental issues.
Landfills dominate thousands of acres, and expensive taxes or tariff barriers are in place to discourage waste from being disposed of on these lands. This technique is quite disadvantageous because plastic takes a long time to self-biodegrade because it uses heat from the sun.
Advanced Technology to Capture CO2
CO2 emissions from industries other than power generation can be captured using technologies such as pre-combustion CO2 capture, post-combustion CO2 capture, oxy-fuel combustion, and chemical looping.
Post-combustion processes such as absorption (using a solvent), adsorption (using a solid material), cryogenic, and membrane separation have been identified as advanced technologies and have been created on a large scale.
The most desirable feature for removing carbon dioxide is absorption with aqueous alkanolamine solvent, which is still the largest manufacturing technology. However, the absorption method has a disadvantage in terms of amine degeneration, hardware corrosion, and the formation of volatile degradation compounds. The solid-material sorption process of CO2 is an option available for amine-based absorption.
Carbon Dioxide Adsorption Method
Solid-material adsorption of carbon dioxide is an approach to amine-based absorption. This is due to the fact that adsorption does have many advantages, including ease of operation, lower power consumption, decent performance for gas and liquid removal, and ease of adsorbent regeneration.
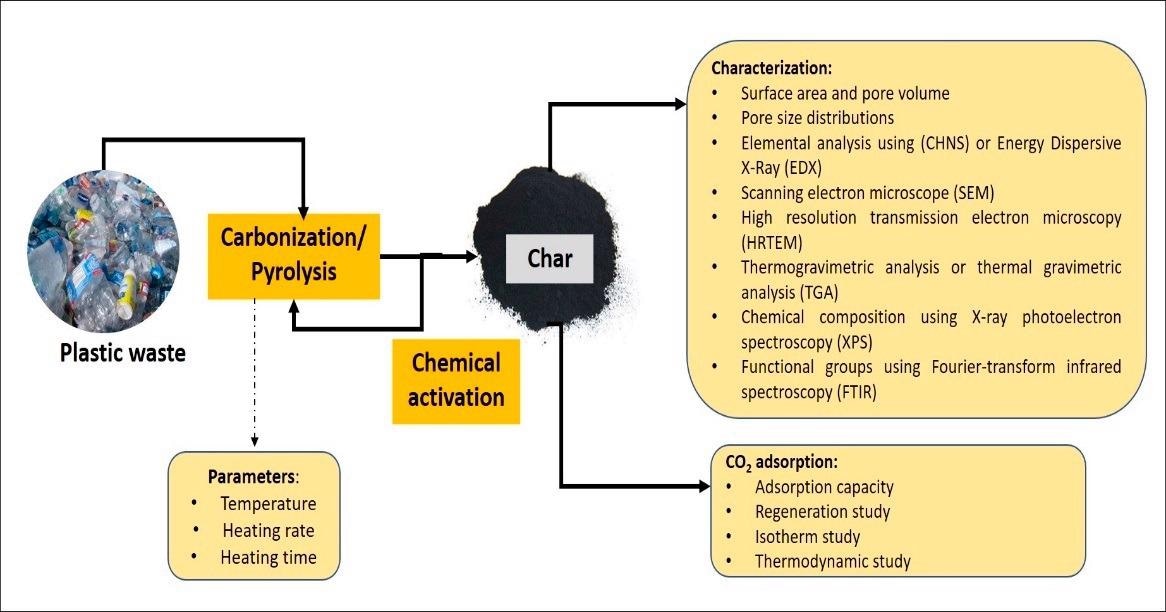
Overall adsorption process using plastic waste as an adsorbent. Image Credit: Hussin, F., et al., Energies
Several enticing adsorbents for CO2 adsorption have been developed, including activated carbon, zeolites, metal-organic frameworks (MOFs), nanoporous silica, a microporous natural polymer, mesoporous carbon substance, and carbon nanotubes. High surface area, high porosity, high stability, excellent recyclability, and high adsorption capacity are the most important properties of adsorbents.
Challenges of Adsorbent Materials
The major challenges of some adsorbent materials, such as MOFs and nanomaterials, are that they are expensive and difficult to develop professionally, making some adsorbents unsuitable for industrial applications.
As a result, more research is required to develop a low-cost substance with a high adsorptive capacity which can be used in mass production. Solid waste is a massive issue in any area of the globe, and converting waste into porous carbon and adsorbents for CO2 capture is an alternative option for reducing waste generation.
Turn Plastics into Porous Carbon
To minimize environmental pollution due to the discharge of used plastic materials, there is increasing awareness of the conversion of plastic waste into useful materials. Multiple researchers have systematically converted plastic waste into porous carbon to be used in a variety of applications, including an organic and inorganic reduction from natural gas implementations, synthetic and natural wastewater, nanoporous adsorbents used as electrode material in supercapacitors, and porous carbon nanosheets with impressive performance used in hydrogen production.
Plastic waste has been converted into porous carbons using a variety of methods, including gasification, direct combustion (physical or chemical activation), hydrothermal carbonization, and pyrolysis. Among the choice of technological development for the treatment of plastic pollution, pyrolysis is one of the most potentials, and the process can be performed with or without catalysts.
Future Research
Most researchers selected the pyrolysis process because of its ability to convert plastic waste into useful products ingredients such as liquid oil and solid char. Meanwhile, there are a number of challenges or limitations to plastic pyrolysis, and more research is needed to address this issue. The most difficult aspect of the plastic waste pyrolysis process is identifying suitable raw material. This is due to the fact that not all plastics are suitable for pyrolysis.
References
Hussin, F., et al. (2021). Transforming Plastic Waste into Porous Carbon for Capturing Carbon Dioxide: A Review. Published: 14 December 2021. Energies 2021, 14(24), 8421; https://www.mdpi.com/1996-1073/14/24/8421
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.