According to a recent paper published in the journal Sustainability, converting remaining algal biomass to value-added products is critical for improving the economics of algae farming. Algal hydrochar formed during hydrothermal treatment is a substance rich in oxygen-containing functional groups and carbon, making it a good option for wastewater cleanup.

Study: Hydrothermal Carbonization of Residual Algal Biomass for Production of Hydrochar as a Biobased Metal Adsorbent. Image Credit: C. Khongchum/ Shutterstock.com
Importance of Algae
The leftover algal biomass obtained after the extraction of important lipids is known as lipid-extracted algae (LEA), and it is high in carbs and proteins. LEA is a byproduct of the algae business that has a mass three times that of the algal lipids collected for biodiesel generation. LEA can be processed to value-added products using hydrothermal carbonization (HTC) to enhance the economics of algae technology for algal lipid-based biofuel generation.
Hydrothermal Carbonization and Hydrochar
Hydrothermal carbonization (HTC) is a hydrothermal process that takes place at low temperatures (180–250 C) and pressures (2–10 MPa). During HTC, algal biomass is subjected to a series of chemical events, which results in a solid substance known as hydrochar, which has a high carbon content and a physiochemical structure comparable to coal. Hydrochar has a lesser contact area but is generated at lower temperatures (hence at a cheaper cost) and is abundant in oxygen-containing functional groups, which allow metal adsorption.
Waste material, municipal waste, cellulosic biofuel, and LEA are all carbonaceous sources that may be transformed into hydrochar. The benefit of LEA is that it will be widely obtainable in the future bioeconomy as a byproduct in huge quantities at algal biorefineries intended to manufacture biofuels from algae lipids.
Hydrochar and Heavy Metal Removal
Heavy metal ions such as zinc and copper are removed from contaminated soils using hydrochar. Low-cost materials are being researched for the removal of heavy metals from different origins of waste and aqueous solutions. Various industrial operations, such as smelting and refining, create large volumes of heavy metal-containing effluent, which must be cleaned before being released into the environment.
Heavy metals are soluble in water and may be absorbed by living creatures, causing serious health problems in marine animals and people. Such wastewaters have already had a deleterious impact on aquatic ecosystems.
Residual Waste Treatment Processes
There are now various ways for treating wastewaters, such as sedimentation and the membrane filtering technique, however they are both expensive. Adsorption is a viable solution that is primarily focused on activated carbon sourced from charcoal and timber. However, activated carbon is expensive, both fossil and renewable.
As biobased adsorbents, a multitude of low-cost hydrochar and biochar derived from algae and other agricultural residues have been investigated. The benefits of biobased adsorbent materials are the abundance of feed, low price, sorption capacity, ease of fabrication, and regeneration capacity. However, because of the high cost of manufacture, they are still in the works.
The current study sought to evaluate the effectiveness of HTC-produced LEA hydrochar for metal cleanup as a way of capitalizing LEA.
Research Findings
It was discovered that reaction time (1–3 h) had no effect on hydrochar production, but temperatures and solids loading did. The LEA hydrochar yield ranged from 26.8 to 36.4 percent and declined practically linearly when the temperature was increased from 180 to 220 C, and the solids loading was reduced from 15 to 8%.
The optimal HTC temperature and materials loading that resulted in the maximum hydrochar production appears to be approximately 180 degrees Celsius and 15%, respectively. Because of lignin's greater resistance to breakdown, lignocellulosic biomass yielded higher hydrochar yields (55–80%).
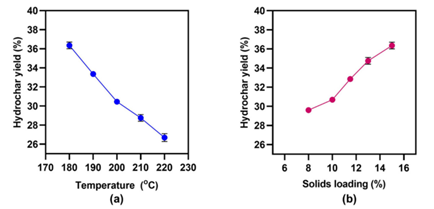
Hydrochar yield after hydrothermal carbonization of lipid-extracted Picochlorum oculatum: (a) Over various temperatures at a constant 15% solids loading for 2 h; (b) over various solid loadings (%) at a constant temperature of 180 °C for 2h. Image Credit: Tsarpali, M et al., Sustainability
The contact area and mean pore diameter of hydrochar samples formed varied from 2.1 to 10.3 m2/g and 2.4 to 7.1 nm, respectively. The considerable increase (5-fold) in the total area of hydrochar with rising sintering temperatures can be attributed to high-temperature thermal pyrolysis.
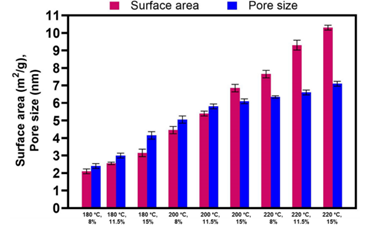
Surface area and average pore size of hydrochar prepared at various HTC temperatures. Image Credit: Tsarpali, M et al., Sustainability
Thermogravimetric analysis (TGA) of LEA and produced hydrochar samples revealed changes in their thermal properties. LEA had a temperature range of 25 to 110 C, whereas hydrochar had a temperature range of 25 to 135 C. Along with this, the decrease in Oxygen concentration from 49.9 to almost 21% while the increase in carbon from 43 to almost 68% was also confirmed by the Elemental analysis technique.
In brief, utilizing hydrothermal carbonization, which is less power consuming than decomposition, lipid-extracted algal biomass was transformed to hydrochar with intriguing metal uptake capabilities. Although theoretically conceivable, this technique can only be economically viable on a wide scale, such as in algal biorefineries. The hydrochar was found to be rich in oxygen-containing functional groups, which improved metal ion sorption when evaluated on synthetic industrial effluent.
Further Reading
Tsarpali, M., Kuhn, J. N. & Philippids, G. P., 2022. Hydrothermal Carbonization of Residual Algal Biomass for Production of Hydrochar as a Biobased Metal Adsorbent. Sustainability, 14(1). 455. Available at: https://www.mdpi.com/2071-1050/14/1/455
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.