A new paper posted to the preprint server Research Square*, in submission to the journal Environmental Science and Pollution Research, has explored the use of rice husk ash as a potentially low-cost, more sustainable source of active silicon dioxide – an essential component in geopolymer production.

Study: Replacement of sodium silicate-based alkali activator by rice husk ash in geopolymer production. Image Credit: Sakdinon Kadchiangsaen/Shutterstock.com
Geopolymers are widely considered to be one of the most promising alternatives to Ordinary Portland Cement (OPC) – a common construction material that exhibits excellent mechanical properties but requires a considerable amount of energy and raw materials to produce, making this highly unsustainable.
As well as requiring a significant amount of energy to produce (thus making it an expensive option), OPC production involves substantial greenhouse gas emissions, meaning that its production is not in line with the current, urgent drive to move to more environmentally friendly and carbon-neutral construction materials.
The centrality of OPC to the construction industry has prompted a great deal of interest, with a number of researchers looking into environmentally-friendly materials which exhibit higher engineering properties than OPC. This work has led to the development of geopolymer as a potential replacement for OPC.
Geopolymer can be understood as an inorganic alumino-silicate powder that is based on geological material. This powder reacts with an alkaline activator to form a binder via a process of polycondensation. Geopolymer exhibits a form that is amorphous to semi-crystalline, featuring three-dimensional Si-O-Al polymeric networks.
A number of complex raw materials have been evaluated for use as the solid component in geopolymer. The paper’s authors cite examples including fly ash, clay, red mud, slag, hydrate lime, and a range of other types of industrial waste.
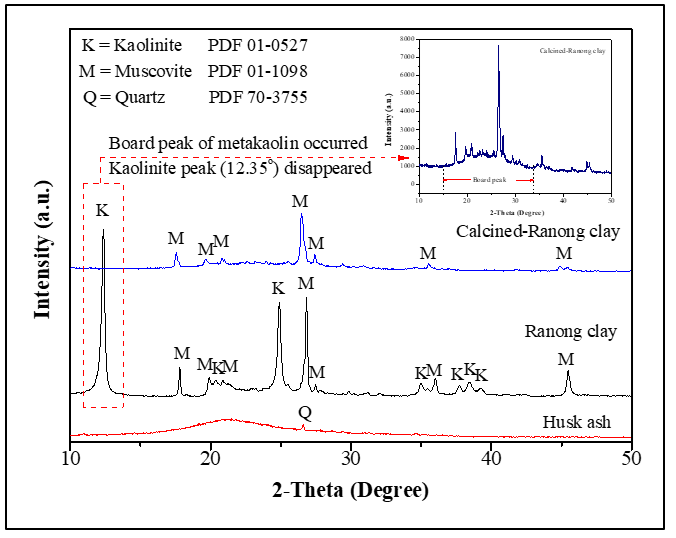
XRD diffraction pattern of starting raw materials after calcined at 750 °C. Image Credit: Siriwan C et al., Research Square
Because clay is an alumino-silicate material possessing a total of 70-90 wt% of Al2O3 and SiO2 as Kaolinite phase, the material’s kaolinite crystal structure can be transformed into a highly reactive amorphous structure of metakaolin. Calcined clay has seen regular use as an alumino-silicate source in clay-based geopolymers.
It was also noted that it is possible to employ a mixture of sodium hydroxide (NaOH) and sodium silicate as an alkali liquid reactant, facilitating the dissolution of alumino-silicate material and delivering a geopolymerization reaction which results in a geopolymer with excellent compressive strength. Unfortunately, this process is complex and can be expensive, meaning it is not ideal for large-scale geopolymer production.
In an effort to move away from industrial waste to other, more affordable materials, agricultural waste has proven to offer excellent promise for geopolymer production due to its high silicon (SiO2) content. One notable example of this – and the focus of the study – is rice husk ash, which has been found to contain between 87 and 95 wt.% of SiO2 depending on the method used to synthesize this.
The rice husk ash is suitable for use as a direct replacement to the aforementioned Calcine clay, representing a viable, environmentally friendly, and more affordable option in the development of geopolymers with high compressive strength.
In order to further test this assertion and optimize the geopolymer properties achievable via the use of rice husk ash, the paper’s authors worked to synthesize sodium silicate from rice husk ash, with a view to employing this in the fabrication of Metakaolin-based geopolymer. These geopolymers were designed to possess SiO2/Al2O3 ratios of 1.58 - 1.95.
Having developed the geopolymers using rice husk ash, these were evaluated in terms of their geopolymerization, compressive strength, microstructure, and structural phases.
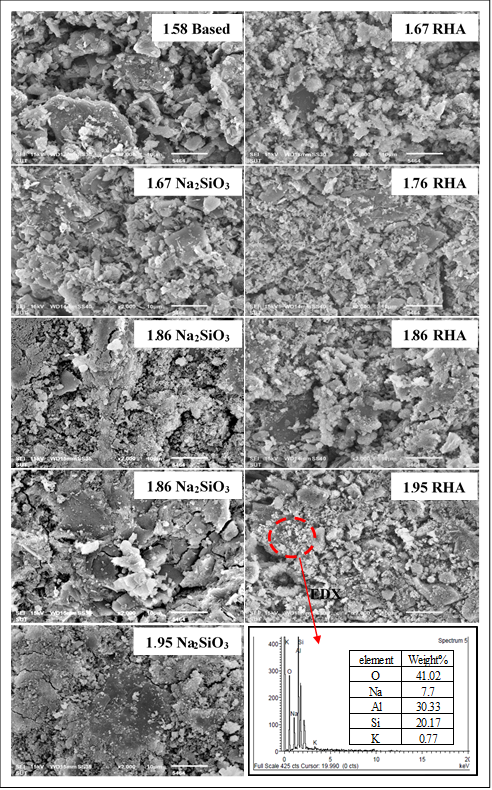
SEM micrograph of geopolymer samples from different resources of active-SiO2 and the ratios of SiO2/Al2O3. Image Credit: Siriwan C et al., Research Square
Their analysis showed that once it had been cured at room temperature for a 24-hour period, the geopolymer with a SiO2/Al2O3 ratio of 1.95 demonstrated excellent compressive strength (25 MPa) – the highest of those evaluated and actually higher than the minimum requirement for OPC.
These findings represent a robust basis for further study and offer a promising means of replacing a vital yet unsustainable component of an essential construction material with a more sustainable alternative.
Not only that, but the substitution of a clay-based material with an agricultural by-product could represent considerable cost savings for manufacturers of these materials while simultaneously improving its mechanical properties.
*Important Notice
Research Square publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Reference
Siriwan Chokkha, Jiratchaya Ayawanna, Anurat Poowancum et al. Replacement of sodium silicate-based alkali activator by rice husk ash in geopolymer production, 18 February 2022, PREPRINT (Version 1) available at Research Square https://www.researchsquare.com/article/rs-1215957/v1
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.