A team of researchers from China and the USA has presented the development of a novel band-aid consisting of a core-shell structure made up of MXene-loaded nanofibers and a hybrid hydrogel that promotes enhanced wound healing. Their research has been published in the journal Bioactive Materials.

Study: An NIR photothermal-responsive hybrid hydrogel for enhanced wound healing. Image Credit: PopTika/Shutterstock.com
Wound Healing and Scar Tissue
When skin tissue is damaged, it can take a while to heal depending on the level of damage. The formation of fibrotic scar tissue is a major problem during would healing. This can lead to loss of function at the wound site. In scarred skin, a fibrotic extracellular matrix can be exhibited, severely affecting the skin’s strength and flexibility. Additionally, scarred tissue can have aesthetic issues for the patient and, depending on the severity of scarring, may affect an individual’s mental health.
When scarring occurs, the scar tissue can become weaker compared to the surrounding skin due to alterations in the epidermal fiber structure. An important clinical goal in research currently is to achieve scarless wound healing. Many strategies have been explored to develop technologies that can promote this.
In wound healing, neovascularization plays an important role. The growth of new blood vessels promotes the proliferation and differentiation of new cells and provides essential nutrients that speed up the repair process. However, if excessive vascularization occurs, it can have a deleterious effect, aggravating tissue deterioration and leading to scar tissue formation.
Additionally, inflammatory responses by the body promote wound healing. Cytokines are produced by various cells in the immune system, which regulate immune function. However, much like excessive vascularization, an excessive inflammatory response by the body causes damage. Achieving the appropriate level of neovascularization and immune response is central to achieving scarless wound tissue healing.
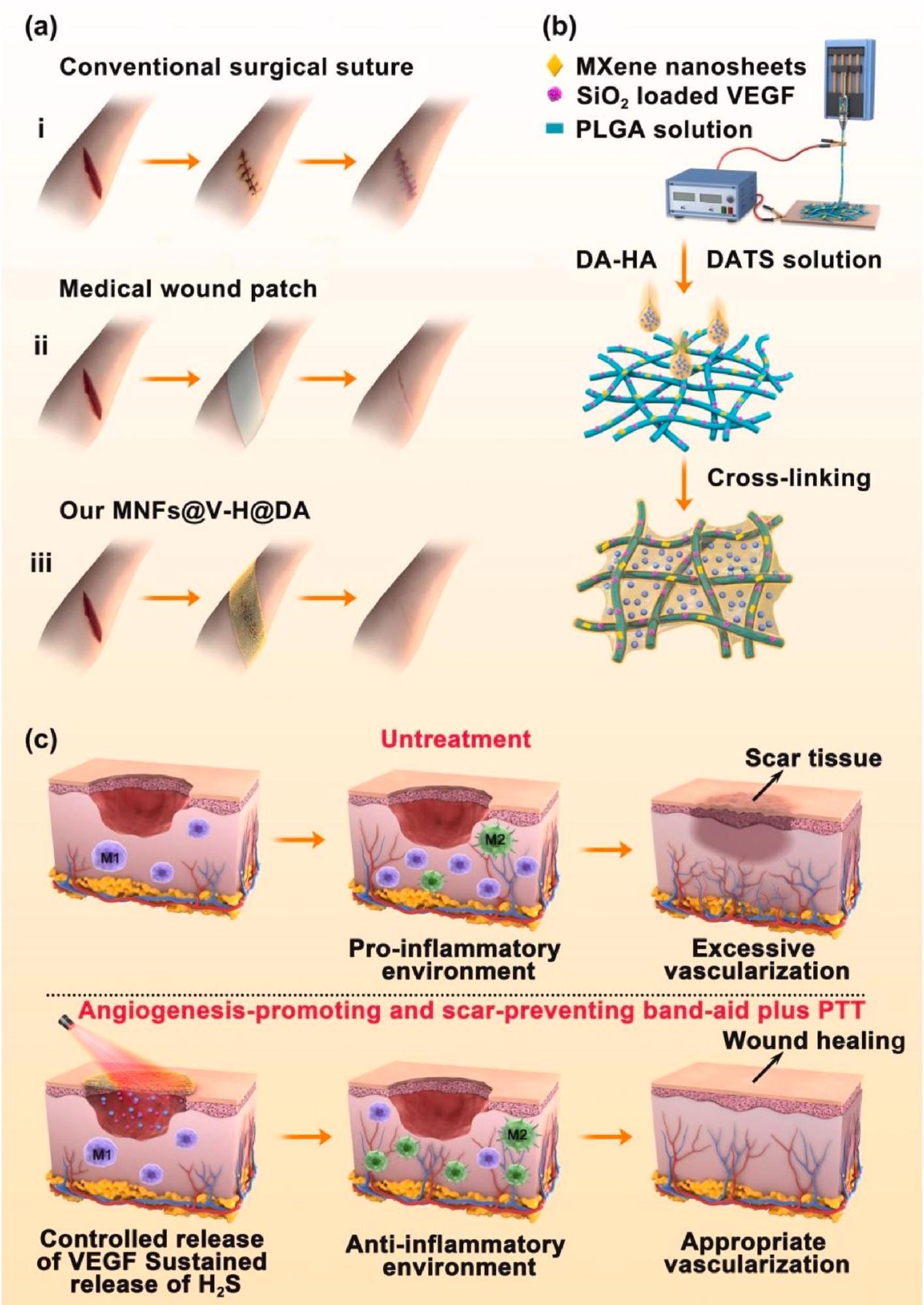
Preparation process, and wound healing process of MNFs@V–H@DA. Various wound healing materials (a), i: traditional surgical suture, ii: medical wound patch, iii: our MNFs@V–H@DA. Scheme of MNFs@V–H@DA fabrication by electrospinning process and surface coating (b). The mechanism of MNFs@V–H@DA on wound healing (c). Image Credit: Jin, L et al., Bioactive Materials
Biomaterials Explored for Wound Healing
In recent studies, several biomaterials have been explored for scarless wound healing-promoting applications. Both hydrogels and nanomaterials have been investigated for this purpose, but their practical application depends on how well they meet the complicated requirements of injured tissues.
An important issue in research is the controlled release of drugs using different scaffolds. Fibers and hydrogels have unique advantages for this purpose. Using fibrous materials as drug carriers is affected by swelling at the fiber interface. Hydrogels, on the other hand, can effectively interface with cells and tissues, facilitating the efficient release of drugs due to their water content. Designing a hybrid system containing both hydrogel and fibers can achieve the controlled release of factors that promote neovascularization and anti-inflammatory responses.
Amongst the various materials explored for this purpose, MXenes have emerged as attractive candidates. Two-dimensional transition metal materials possess unique physical and chemical properties. An especially attractive property that has made them the focus of recent research in biomedical science is their high photothermal conversion efficiency.
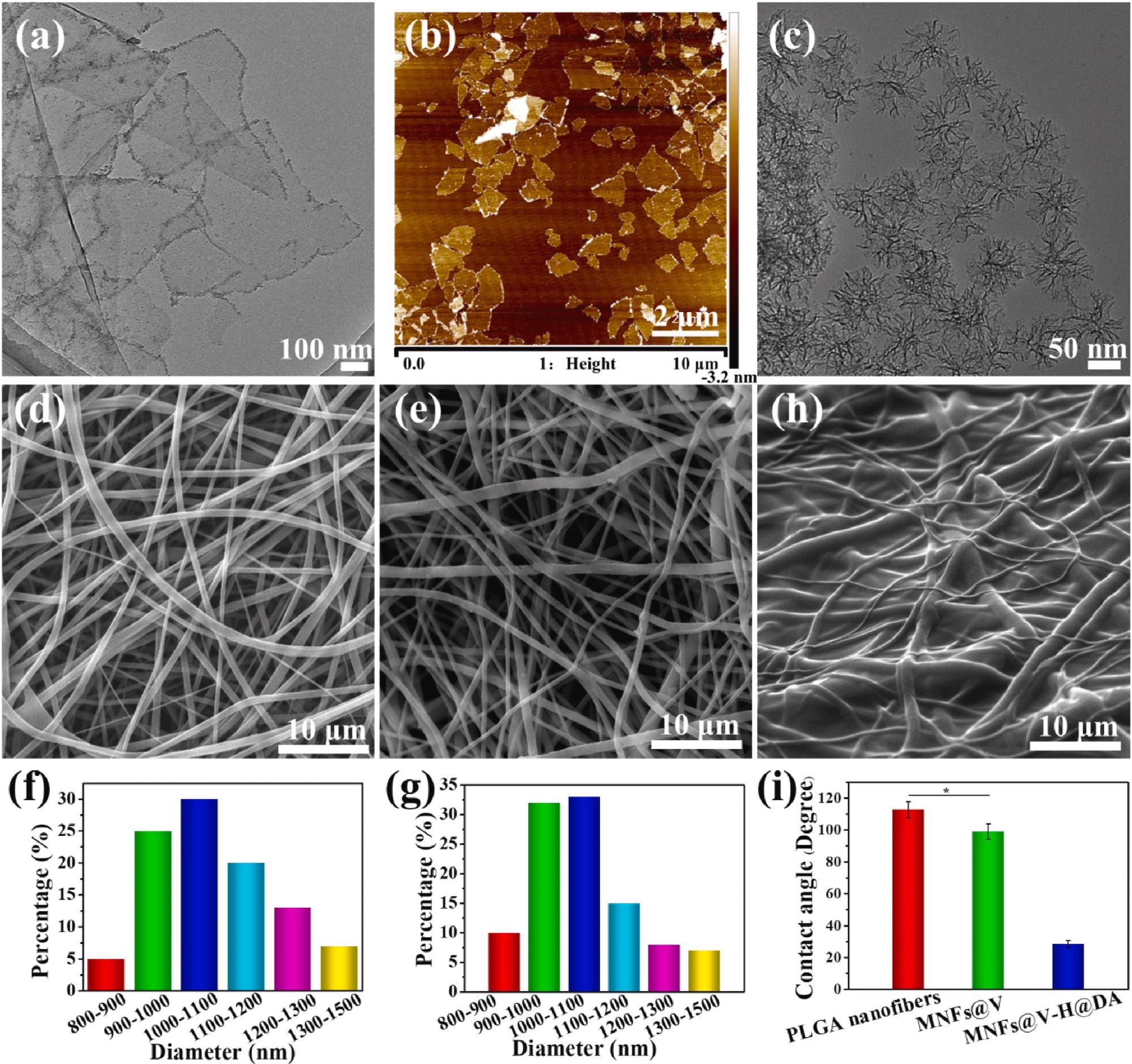
Morphology characterization of MXene nanosheets, SiO2 NPs and MNFs@V–H@DA. TEM and AFM images of MXene nanosheets (a, b). TEM image of SiO2 NPs (c). SEM images of PLGA (d), MNFs@V (e), MNFs@V–H@DA (f). Diameter distribution of PLGA (f) and MNFs@V (g). The SEM image of MNFs@V–H@DA (h). The contact angels of PLGA nanofibers, MNFs@V, MNFs@V–H@DA (i). Data are presented as mean ± standard deviation, n = 3, *p value < 0.05. Image Credit: Jin, L et al., Bioactive Materials
The Study
Based on the photothermal conversion efficiency of MXenes, the team from China and the USA have developed a novel near-infrared-responsive band-aid which promotes scarless tissue repair. The technology combines the advantages of hydrogels and nanofibers.
The band-aid consists of a core-shell structure consisting of MXene-loaded PLGA nanofibers with a dopamine-hyaluronic acid hydrogel shell. The novel band-aid developed by the researchers encapsulates vascular endothelial growth factor and a H2S donor, diallyl trisulfide. H2S promotes the polarization of macrophages into the anti-inflammatory M2 phenotype, which inhibits the excessive proliferation of fibroblasts and the deposit of extracellular matrix during the late stage of healing.
The release of vascular endothelial growth factor is controlled in the band-aid by adjusting the time of near-infrared exposure in an “on/off” manner. This prevents excessive vascularization and the deposition of extracellular matrix at the wound site, again inhibiting the formation of scar tissue at the wound site.
Immunohistochemical and H&E staining of spleen tissue demonstrated that the system remodeled the wound site’s immune microenvironment. The results of staining indicated that the treatment promoted the secretion of IL-4, an anti-inflammatory factor, and reduced the proliferation of IFN-γ, a pro-inflammatory factor.
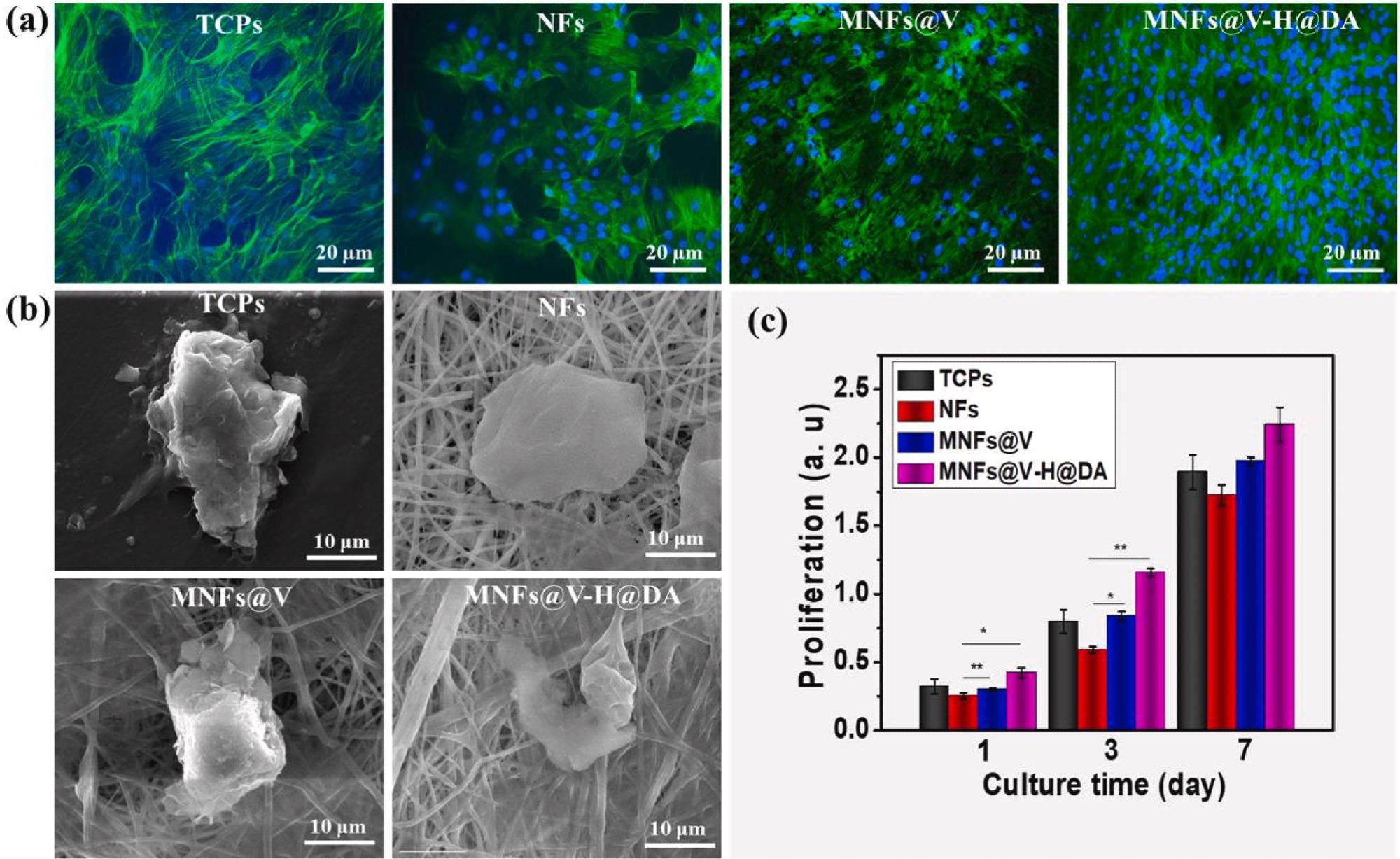
Biocompatibility of MNFs@V–H@DA. Fluorescent images (a) and cellular morphology (b) of MSCs cultured 5 days on TCPs, PLGA nanofibers, MNFs@V and MNFs@V–H@DA. The cellular proliferation (c) of MSCs cultured on TCPs, NFs, MNFs@V and MNFs@V–H@DA with 7 days period. Data are presented as mean ± standard deviation, n = 3, *p value < 0.05, **p value < 0.01. Image Credit: Jin, L et al., Bioactive Materials
The spleen was chosen as it is the main peripheral immune regulatory organ that secretes factors that regulate the immune response. Thus, the treatment changes the immune microenvironment from a pro-inflammatory to an anti-inflammatory one. Additionally, several other factors were categorized, including biocompatibility, cellular cytotoxicity, activity, and morphology.
Based on the results of their findings, the authors have stated that their novel MXene-loaded nanofiber/dopamine-hyaluronic acid hydrogel near-infrared responsive band-aid is effective at promoting neovascularization and inhibiting inflammatory responses. The proposed treatment successfully promotes scarless wound healing, making it an attractive candidate for clinical applications.
Further Reading
Jin, L et al. (2022) An NIR photothermal-responsive hybrid hydrogel for enhanced wound healing [online] Bioactive Materials 2022 | sciencedirect.com. Available at: https://www.sciencedirect.com/science/article/pii/S2452199X22001220
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.