Jumping the queue is more often than not considered rude, but at times it is acceptable. This is very true, especially in the case of salt.
 Common salt (NaCl) acts as an intermediary in the chemical vapor deposition growth of 2D molybdenum disulfide, speeding the process of its creation. Materials theorists at Rice University discovered that salt and a precursor form a eutectic, which has a lower melting temperature than either of them. Image Credit: Jincheng Lei/Yakobson Research Group.
Common salt (NaCl) acts as an intermediary in the chemical vapor deposition growth of 2D molybdenum disulfide, speeding the process of its creation. Materials theorists at Rice University discovered that salt and a precursor form a eutectic, which has a lower melting temperature than either of them. Image Credit: Jincheng Lei/Yakobson Research Group.
Boris Yakobson, a materials theorist from the Rice University laboratory, has displayed why in its follow-up to a 2018 study that illustrated how salt streamlines the creation of useful 2D molybdenum disulfide (MoS2) using a first-principles analysis of the process that could be able to refine it more.
The theoretical study performed by Yakobson and collaborators Jincheng Lei, Yu Xie, and Alex Kutana, all alumni of his laboratory and researcher Ksenia Bets, displays why salt, especially iodized salt, tends to reduce the reaction temperature in a chemical vapor deposition (CVD) furnace considered essential to develop MoS2 via the simulation of atom-level energies.
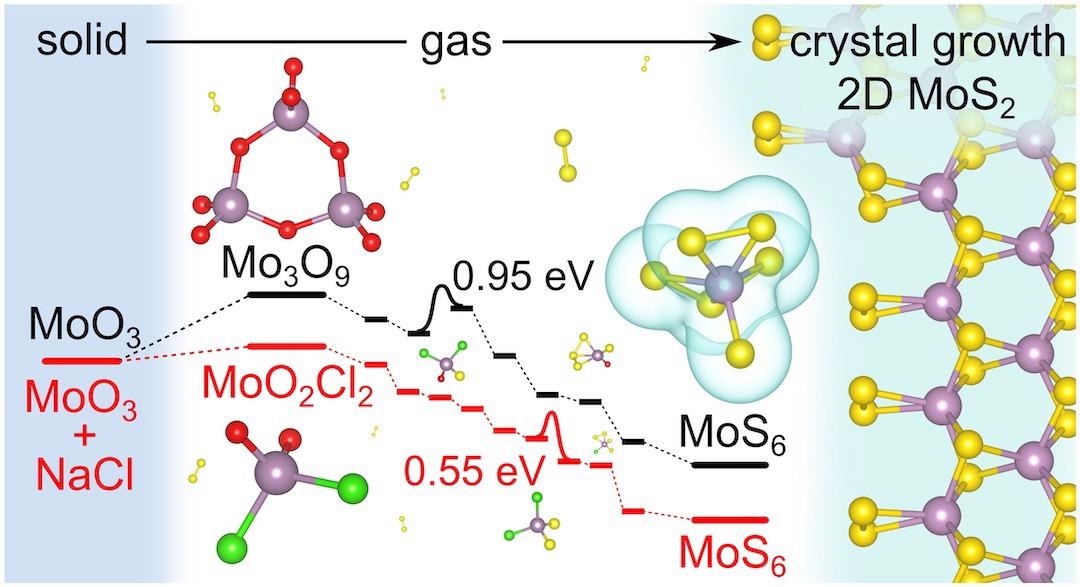
Salt does so by helping to skip a few steps and jump high energy barriers in traditional CVD growth to provide much more MoS6. It is considered a necessary precursor to 2D MoS2.
Their study, published in the Journal of the American Chemical Society, concentrated on how salt reduces activation barriers to improve the sulfurization of molybdenum oxyhalides, which is the gas feedstock in MoS2 crystallization.
MoS2 is known to be a natural compound available in huge forms in the name of molybdenite. Also, in 2D form, it is highly desired for its semiconducting properties, which provides hope for advances in the field of optoelectronics, electronic, spintronic, catalytic and medical applications. However, 2D MoS2 remains difficult to manufacture in commercial amounts.
Initially, the Rice group entered the fray when laboratories in Singapore, China, Japan and Taiwan made use of salt to make a so-called “library” of 2D materials that integrated transition metals and chalcogens.
The good functioning of the materials - even theoretical ones - was a puzzle, thereby urging them to call on the Yakobson laboratory’s expertise in modeling materials.
Their extensive models display that while the international laboratories utilized chloride salts to create their library of materials, the iodide salts generally found on kitchen tables are better at expediting the MoS2 synthesis.
Fast and large-scale synthesis is imperative for the widespread application of MoS2. We carefully studied the entire growth process, hoping to optimize it as much as possible. It turned out that by simply changing chloride to iodide, one could synthesize MoS2 much faster while at even lower growth temperatures.
Jincheng Lei, Department of Materials Science & Nanoengineering, Rice University
This occurs when salt and the precursor combine to develop a eutectic, a blend of substances that melt and solidify at a single temperature that is lower compared to the constituents melting point.
After salt-assisted synthesis was shown to enable the growth of many more TMD (transition metal dichalcogenide) compounds than was possible beforehand and significantly improved growth conditions for previously synthesized ones, it became clear that there is something special about this process.
Ksenia V. Bets, Department of Materials Science & Nanoengineering, Rice University
Bets added, “Some experimental groups attempted to investigate further, but monitoring the molecular composition of the gas phase under growth conditions is not a simple task. Even then, you cannot see the whole picture.”
“We were very thorough, following up on Jincheng’s work on the mechanism of conventional MoS2 growth. We simulated all parts of the process, from sulfurization to the 2D crystal growth. This comprehensive approach paid off,” added Bets.
In simulations, the research group from Rice was able to directly note the complete sulfurization process as there was a gradual replacement of chlorine and oxygen atoms by sulfur in MoO2Cl2, a general precursor, under CVD conditions.
The laboratory stated that the eutectic effect might be considered a common phenomenon in the CVD synthesis of 2D dichalcogenide monolayers, and hence worth continued study.
Lei is currently working as a postdoctoral researcher at Yale University. Xie is, at present, a professor at Xi’an University, China. Kutana is an assistant professor at Nagoya University, Japan. Yakobson is the Karl F. Hasselmann Professor of Engineering and a professor of materials science and nanoengineering and also of chemistry
This study was financially supported by the Department of Energy, Basic Energy Sciences (DE-SC0012547), and the Welch Foundation (C-1590).
Journal Reference:
Lei, J., et al. (2022) Salt-Assisted MoS2 Growth: Molecular Mechanisms from the First Principles. Journal of American Chemical Society. doi.org/10.1021/jacs.2c02497.