A new paper in the journal Energies has investigated the encapsulation of lithium atoms in different-sized spherical fullerenes.
Study: An Analytical Model for Lithium Storage in Spherical Fullerenes. Image Credit: Emmily/Shutterstock.com
Carbon Fullerenes: An Overview
Carbon is an extremely abundant natural element, which is fundamental to organic processes and organic chemistry. Elemental carbon can be easily produced using conversion reactions, and a multitude of carbon-based materials have been synthesized over the past few decades.
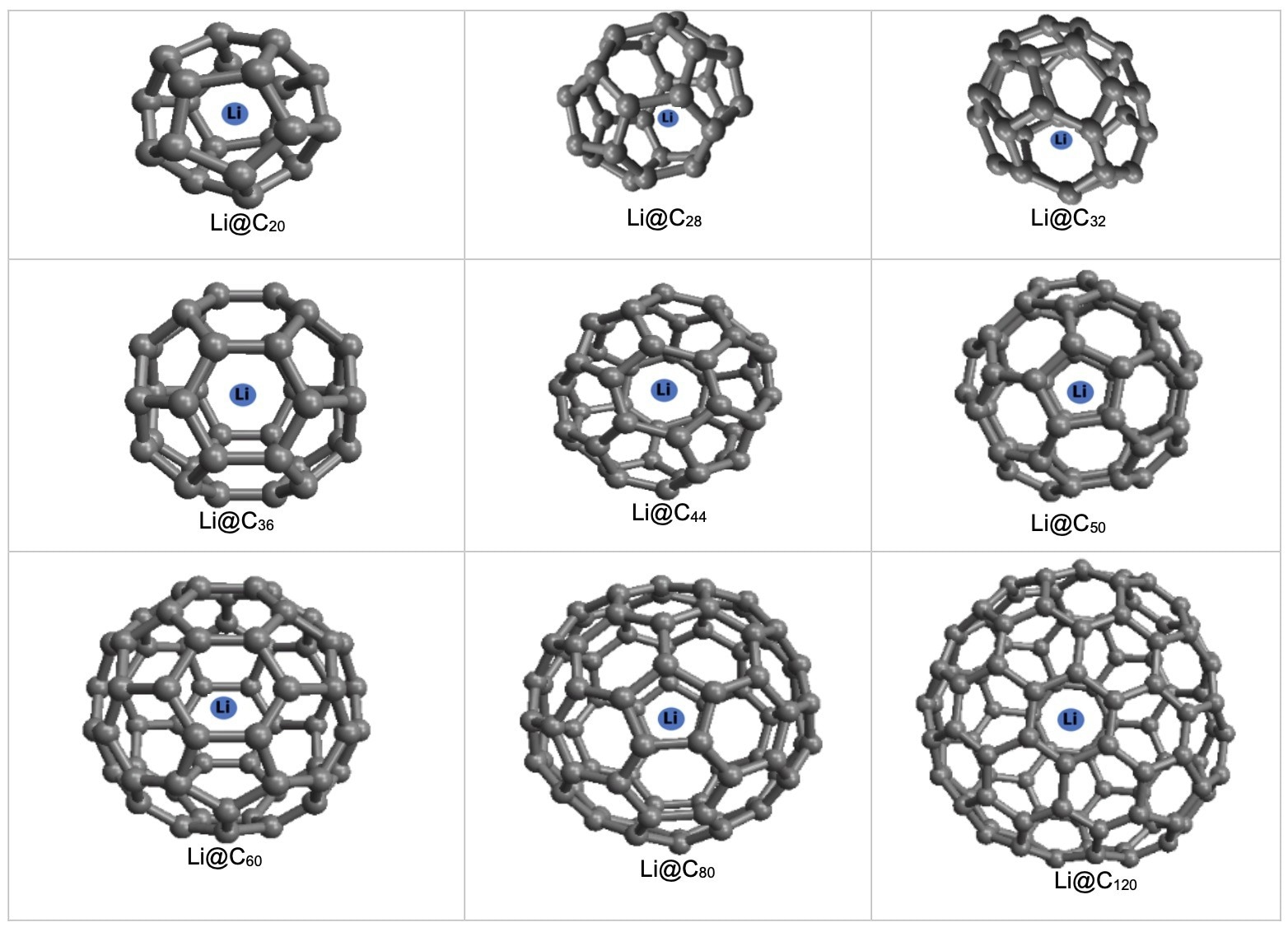
Storage of a Li atom in CN fullerenes. Image Credit: Alshehri, M.A et al., Energies
Carbon nanomaterials have become the focus of research interest in multiple scientific fields, with lab-based studies and industry producing numerous commercial products. Carbon nanotubes, nano-onions, nanobundles, nanosheets, nanotori, and fullerenes have garnered significant research attention, with many studies exploring their properties and potential applications.
Fullerenes were discovered in 1985, and since their initial discovery have been investigated for their application in the fields of biomedicine, semiconductors, and electronics. Fullerenes have large surface-to-volume ratios, which play a key role in their electronic properties, and van der Waals forces in these materials contribute to their mechanical properties.
Fullerenes are excellent electron acceptors, antioxidants, and radical scavengers, which has made them revolutionary materials in several innovative technologies. Furthermore, fullerene research may help to create further novel types of nano-scaled devices in the future.
Encapsulating Lithium in Fullerenes
Lithium ions are highly chemically reactive, which requires stringent regulations to ensure their safe processing, handling, utilization, and conservation to avoid potential environmental harm arising from their use. The increasing use of lithium in key sustainable technologies such as batteries has facilitated urgent research into strategies that can mitigate any potential environmental damage.
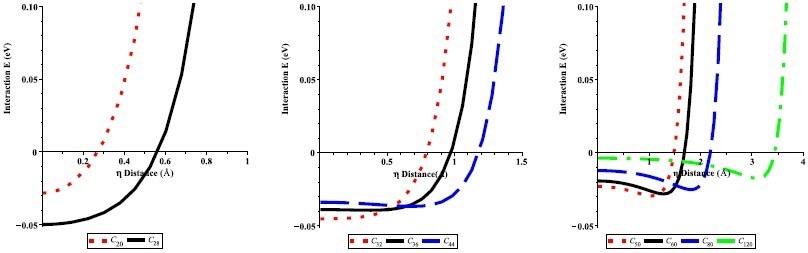
Interaction energies of an offset Li atom inside the CN fullerenes (N = 20, 28, 32, 36, 44, 50, 60, 80, and 120) with respect to the radial offset distance η from the center of the fullerenes. Image Credit: Alshehri, M.A et al., Energies
Carbon nanomaterials have emerged as candidate materials to meet this crucial requirement of rechargeable lithium battery research. Beneficial properties include their high specific surface area, excellent electrical conductivity, good thermal stability, low cost, environmental benignity, and hierarchical architecture.
Carbon fullerenes containing lithium ions and atoms could be considered as novel anode materials due to the existence of strong covalent bonds with low ionicity. This characteristic supports large bandwidths. Other attractive properties of these nanomaterials include high lithium capacities, small volume changes during lithium extraction and insertion, fast extraction/insertion kinetics, and high chemical potentials.
Many studies recently have focused on lithium encapsulation in carbon fullerenes and improving cyclic performance during battery recharging. Studies have employed analytical methods such as DFT calculations, photoelectron spectroscopy, and scanning tunneling microscopy to investigate the electrical, geometric, and thermodynamic properties of carbon fullerene/lithium nanomaterials.
The Paper
In the paper, lithium atom-containing spherical fullerenes were produced to evaluate their properties and interactions. Varying sizes of fullerenes were investigated. Interaction energies between lithium atoms and fullerenes were modeled by exploiting the continuum approximation and the 6-12 LJ potential function. Classical applied mathematics and mechanics were employed to adopt these functions.
Numerical results for different-sized fullerenes were yielded by the theoretical method proposed in the research. It was demonstrated that lithium atoms can be encapsulated in fullerenes with a radius greater than 2 Å, with the preferred radius being approximately 2.4 Å. Fullerene/lithium atom complexes at this radius possess minimal activation energy.
The results of this study are in good agreement with previous studies which proposed the encapsulation of lithium ions in different-sized carbon fullerenes, where N ≥ 20. The different fullerene sizes investigated in the research for lithium storage were from N = 20 to 120. Maple software was used to carry out the calculations in conjunction with the model’s physical parameters.
The study’s methods and results provide the theoretical design of lithium-atom-containing fullerenes, which can be considered to be novel anode materials in rechargeable batteries. Future research should focus on investigating any changes in electronic structures during adsorption and the adsorption energies. The interaction between lithium atoms and other nanomaterials should also be evaluated.
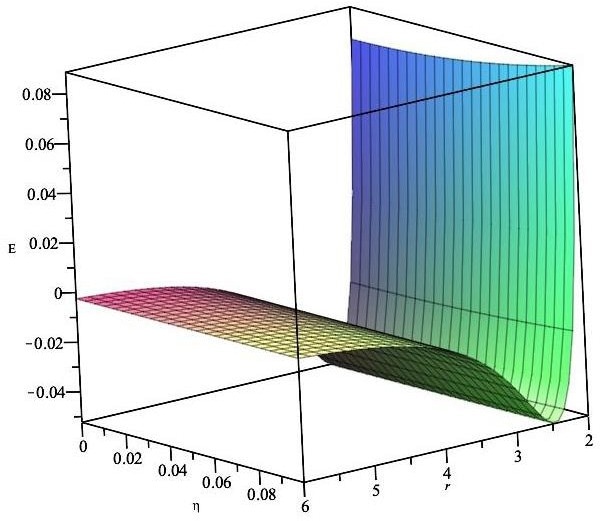
Total energy for a Li atom stored inside a CN fullerene. Image Credit: Alshehri, M.A et al., Energies
In Summary
The new paper in Energies has provided an in-depth investigation of encapsulating lithium atoms in carbon fullerenes of different sizes and the interactions between these two materials. By employing the LJ function and continuum approximation, the van der Waals interactions between fullerene and lithium atoms can be approximated.
Mathematical modeling was employed to produce the theoretical design of an optimal fullerene configuration for lithium atom encapsulation. Whilst more work is needed in this area of research, the paper has provided a sound basis for future studies into the encapsulation of lithium atoms in carbon fullerenes and other nanomaterials and the design of suitable anodes for advanced batteries and materials for other key areas of scientific research.
Further Reading
Alshehri, M.A (2022) An Analytical Model for Lithium Storage in Spherical Fullerenes Energies 15(19) 7154 [online] mdpi.com. Available at: https://www.mdpi.com/1996-1073/15/19/7154
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.