The discovery of Ba2LuAlO5 as a promising proton conductor paints a bright future for protonic ceramic fuel cells, report scientists from Tokyo Tech. Experiments show that this novel material has a remarkably high proton conductivity even without any additional chemical modifications, and molecular dynamics simulations reveal the underlying reasons. These new insights may pave the way to safer and more efficient energy technologies.
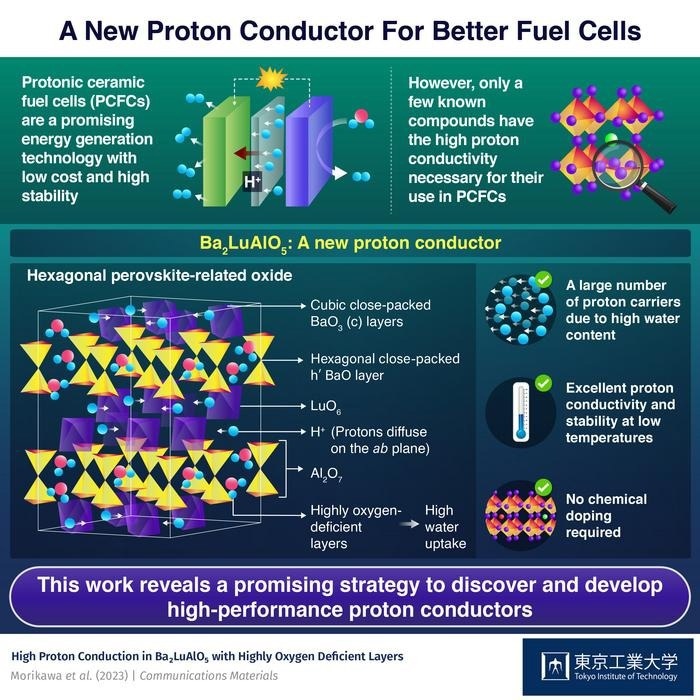
Image Credit: Prof.M.Yashima, Tokyo Institute of Technology
When talking about sustainability, the ways in which a society generates energy are some of the most important factors of consideration. Eager to eventually replace traditional energy sources such as coal and oil, scientists across the world are trying to develop environmentally friendly technologies that produce energy safely and more efficiently. Among them, fuel cells have been steadily gaining traction since the 1960s as a promising approach to producing electricity directly from electrochemical reactions.
However, typical fuel cells based on solid oxides have a notable drawback in that they operate at high temperatures, usually over 700 °C. That is why many scientists have focused on protonic ceramic fuel cells (PCFCs) instead. These cells use special ceramics that conduct protons (H+) instead of oxide anions (O2−). Thanks to a much lower operating temperature in the range of 300 to 600 °C, PCFCs can ensure a stable energy supply at a lower cost, compared to most other fuel cells. Unfortunately, only a few proton-conducting materials with reasonable performance are currently known, which is slowing down progress in this field.
To address this challenge, a team of researchers, including Professor Masatomo Yashima from Tokyo Institute of Technology (Tokyo Tech) in Japan, has been on the lookout for good proton conductor candidates for PCFCs. In their latest study, published in Communications Materials, the team reported the remarkable properties of Ba2LuAlO5, a new hexagonal perovskite-related oxide that has provided interesting insights into proton conduction.
Prof. Yashima and colleagues discovered Ba2LuAlO5 while focusing on finding compounds with a lot of intrinsic oxygen vacancies. This was motivated by the results of previous studies highlighting the importance of these vacancies in proton conduction. Experiments on Ba2LuAlO5 samples revealed that this material has a high proton conductivity in its bulk at low temperatures—its conductivity was 10‒2 S cm‒1 at 487 °C and 1.5×10‒3 S cm‒1 at 232 °C—even without additional chemical refinements such as doping.
Afterwards, the team sought to find out the underlying reasons for this property. Through molecular dynamics simulations and neutron diffraction measurements, they learned two important characteristics of Ba2LuAlO5. The first is that this oxide absorbs a lot of water (H2O), compared to other similar materials, to form Ba2LuAlO5.0.5H2O. This large water uptake, which occurs within two opposing layers of AlO4 tetrahedra, is made possible by a high number of intrinsic oxygen vacancies in the hexagonal close-packed h’ BaO layers. In turn, the oxide’s higher water content increases its proton conductivity through various mechanisms, such as higher proton concentration and enhanced proton hopping.
The second important characteristic is related to how protons move through Ba2LuAlO5. Simulations revealed that protons diffuse mainly along the interfaces of LuO6 layers, which form cubic close-packed c BaO3 layers, rather than through the AlO4 layers. This information could be critical in the search for other proton conducting materials, as Prof. Yashima explains: “Our work provides new design guidelines that open up unexplored avenues for the development of higher-performance proton conductors in the future.”
The researchers expect to find other proton-conducting materials based on Ba2LuAlO5 in upcoming studies. “By modifying the chemical composition of Ba2LuAlO5, further improvements in proton conductivity can be expected,” comments Prof. Yashima, “For example, the perovskite-related oxide Ba2InAlO5 may also exhibit high conductivity since its structure is quite similar to that of Ba2LuAlO5.”
Overall, the future of PCFCs seems bright and, by extension, so does the future of sustainable energy generation technologies.