Chinese researchers have made a notable advancement in enhancing the salt stability of hydrogels used in sodium-ion batteries, which are commonly found in flexible aqueous batteries used in portable electronics. They achieved this through a chemical modification inspired by nature.
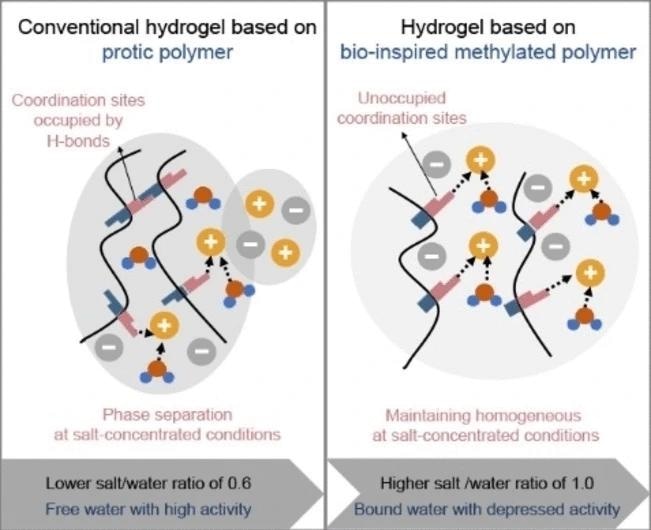
Image Credit: Angewandte Chemie
By simply methylating the structural polymer of the hydrogel, they effectively prevented salt precipitation, leading to improved battery capacity and cycling performance. This achievement has been reported in the journal Angewandte Chemie.
Sodium-ion batteries offer a promising alternative to lithium-ion batteries due to their use of cost-effective and environmentally friendly materials. However, the development of sodium-ion batteries necessitates the creation of numerous new components, all tailored to accommodate sodium ions.
Among these components, the electrolyte plays a pivotal role. In the context of thin and flexible batteries, the electrolyte often takes the form of a hydrogel. These pliable materials, which contain water, have the capacity to absorb dissolved sodium salts and facilitate ion conduction.
While hydrogels have proven to be suitable for various applications, a persisting challenge has been the occurrence of phase separation and salt precipitation at elevated salt concentrations required for achieving a wide electrochemical stability range.
A team led by Guanglei Cui, working at the Chinese Academy of Sciences in Qingdao, China, has achieved a breakthrough by modifying a hydrogel designed for use in sodium-ion batteries. This modification has significantly enhanced the hydrogel's ability to absorb a larger amount of salt while maintaining stability and security within the battery system.
To achieve this enhancement, the researchers applied a technique inspired by natural mechanisms used in large biomolecules for regulating water and salt binding: methylation. In proteins, methylation results in the “capping” of amine and amide groups, making them less accessible to water molecules, which in turn affects their involvement in cross-linking within the protein’s structure and the dissolution of salt ions.
As polyamide polymers utilized in hydrogels contain amide groups, their substantial cross-linking via water molecules can result in salting-out, resulting in electrolyte degradation. To address this issue, the team conducted a comparison between a hydrogel comprised of a typical polyamide and one composed of a polyamide with amide groups that had undergone methylation.
The methylated amide variant exhibited a considerably higher capacity to absorb salt than the original one. Even when subjected to exceptionally high salt concentrations, the hydrogel electrolyte remained both transparent and stable.
The increased salt concentration expands the electrochemically usable voltage range of the cell. Furthermore, the team observed no indications of electrode deterioration, resulting in improved cycling stability. The assembled battery cell exhibited a higher capacity compared to the non-methylated version. Surprisingly, inexpensive aluminum foil could be employed as a current collector in this setup.
The authors propose that the straightforward methylation of polyamide could also find application in other technologies. For instance, it could enhance the resistance of hydrogels to salts, thereby increasing their overall stability. This innovation may have potential applications in areas such as drug development.
Journal Reference
Liu, T., et al. (2023). A Bio‐Inspired Methylation Approach to Salt‐Concentrated Hydrogel Electrolytes for Long‐Life Rechargeable Batteries. Angewandte Chemie. doi.org/10.1002/anie.202311589.