Several shortcomings of sodium-ion batteries can be addressed by improving the battery's composition. Doping the cathode material with foreign elements is one option. Now, a group from HZB and Humboldt-Universität zu Berlin has studied the consequences of doping with magnesium and scandium.
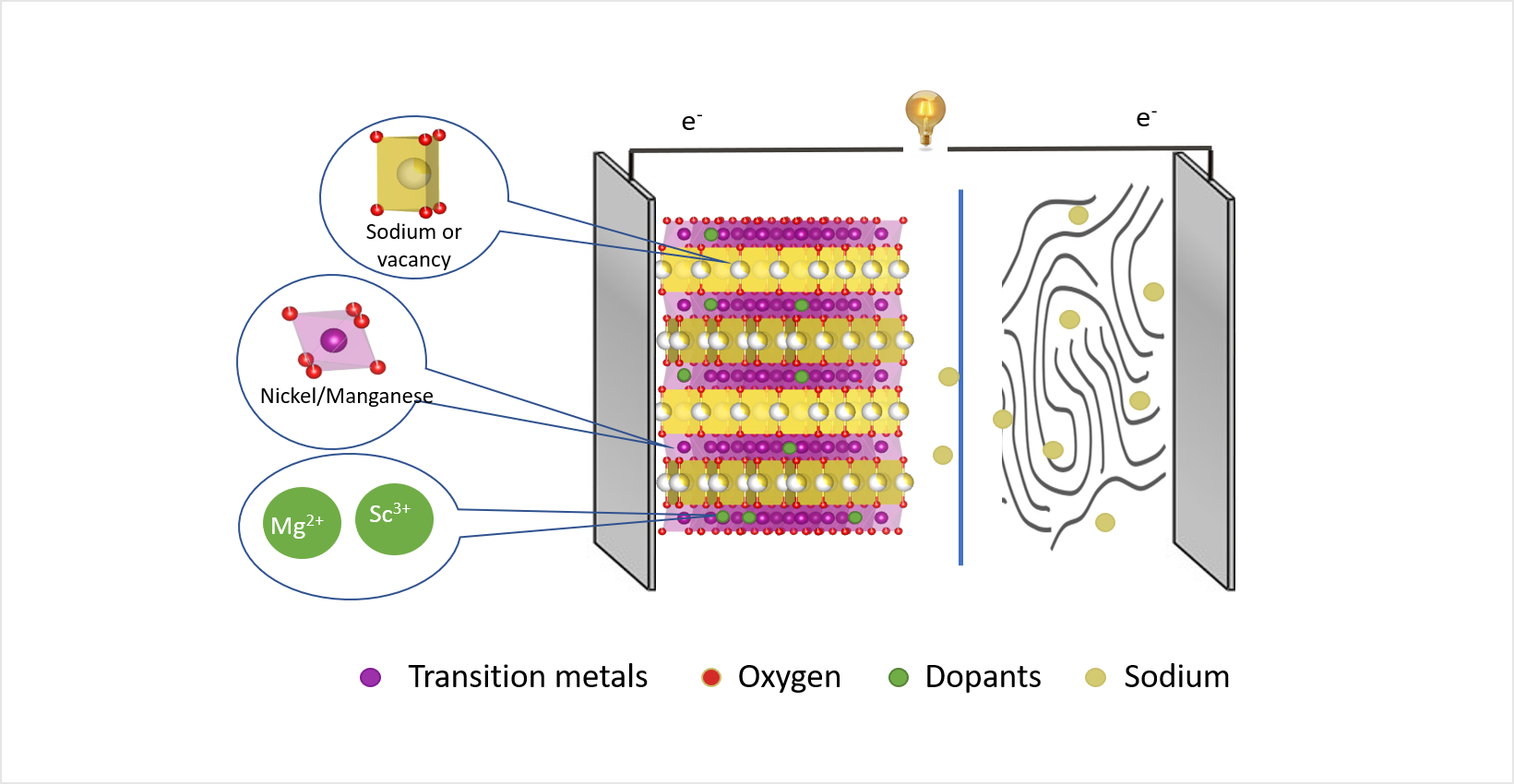 The schematic illustration shows a sodium ion battery: The positive electrode or cathode (left) consists of layered transition metal oxides which form a host structure for sodium ions. The transition metal nickel can be replaced either by magnesium or scandium ions. Image Credit: Helmholtz-Zentrum Berlin
The schematic illustration shows a sodium ion battery: The positive electrode or cathode (left) consists of layered transition metal oxides which form a host structure for sodium ions. The transition metal nickel can be replaced either by magnesium or scandium ions. Image Credit: Helmholtz-Zentrum Berlin
To provide a comprehensive picture, the scientists gathered data from the X-Ray sources BESSY II, PETRA III, and SOLARIS. They discovered two conflicting mechanisms that control the stability of the cathodes.
The highest energy density per kg is achieved by lithium-ion batteries (LIBs), yet lithium resources are scarce. On the other hand, sodium is the second-best choice in terms of energy density and has an almost limitless supply. Therefore, sodium-ion batteries (SIBs) might be a good substitute, particularly in situations where the batteries' weight is not a big concern, like in stationary energy storage systems.
Experts, however, are certain that a focused material design for the cathodes might greatly boost the capacity of these batteries. Particularly promising cathode materials are those composed of layered transition metal oxides containing the metals manganese and nickel (NMO cathodes). During discharge, the sodium ions are held in these host structures, and during charging, they are released.
However, there is a chance that chemical reactions will occur, which could increase capacity at first but eventually cause local structural changes that would deteriorate the cathode material. This ultimately results in a shorter lifespan for the sodium-ion batteries.
Dr. Katherine Mazzio said, “But we need high capacity with high stability.”
He is also a member of the joint research group “Operando Battery Analysis” at HZB and the Humboldt-Universität zu Berlin, headed by Professor Philipp Adelhelm.
They have now looked into how doping with foreign elements impacts the NMO cathodes, led by PhD Student Yongchun Li. A variety of elements, such as magnesium (Mg 2+) or scandium (Sc 3+), with ionic radii comparable to nickel (Ni 2+) but distinct valence states, were chosen as dopants.
Three Years of Experiments at BESSY II, PETRA III, and SOLARIS
They needed to conduct experiments at three distinct X-Ray sources to determine the relative contributions of the two components. Resonant inelastic X-Ray scattering (RIXS) and X-Ray absorption spectroscopy (XAS) were used at BESSY II to analyze the samples in the soft and hard X-Ray ranges.
At PETRA III, XRD and pair distribution function analysis (PDF) with hard X-Rays were used to assess structural changes. Finally, additional soft XAS investigations were conducted at the PIRX beamline at SOLARIS to gain more in-depth knowledge of the element magnesium.
Scandium Does Not Improve Stability
Mazzio explained, “The results surprised us.”
Scandium doping does not increase stability while causing fewer structural alterations during the electrochemical cycle than magnesium doping.
Until now, it was thought that suppressing phase transitions (and thus volume changes) would also improve the cathode material cycling performance over many cycles. But that is not enough.
Katherine A. Mazzio, Institute for Chemistry, Humboldt-University Berlin
It All Depends on the Ratio
In NMO, magnesium doping further inhibits the oxygen redox process. This was particularly surprising because stacked manganese oxides are known to undergo an oxygen redox reaction when magnesium is present.
We analyzed different Mg/Ni ratios in NMO and found that the oxygen redox reaction reaches a minimum at a ratio close to 1, and only through a combination of advanced X-ray techniques could we show that it is more than just suppression phase transitions that is important for improving the long-term cycling behavior, but also the interplay between Ni and O redox activity dictate performance.
Katherine A. Mazzio, Institute for Chemistry, Humboldt-University Berlin
Journal Reference:
Li, Y., et al. (2024) Competing Mechanisms Determine Oxygen Redox in Doped Ni–Mn Based Layered Oxides for Na-Ion Batteries. Advanced Materials. doi.org/10.1002/adma.202309842.