Ethyl crotonate is an organic chemical compound used as a plasticizer for acrylic resins and also as a solvent for cellulose esters. it is clear and colourless at room temperature and has a pungent odour. It is also soluble in water.
Figure 1 shows the 1HNMR spectrum of 25% ethyl crotonate in CDCl3. It took just 7 seconds to record this spectrum in a single scan. Both 1H-1H couplings and peaks are suitably resolved and hence could be assigned to the molecular structure.
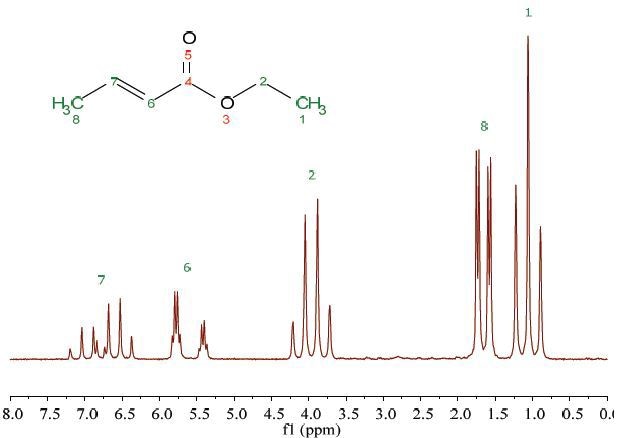
Figure 1. Proton NMR spectrum of 25% ethyl crotonate in CDCl3.
1H NMR Relaxation
Figures 2, 3 and 4 depict the relaxation time measurements. Both T1 and T2 values of the exchanging hydroxyl proton can be seen in the spectrum. The relaxation times are shortest and longest for the CH3 protons and CH protons, respectively. The first data point amplitude increases with the number of protons for the related peak.
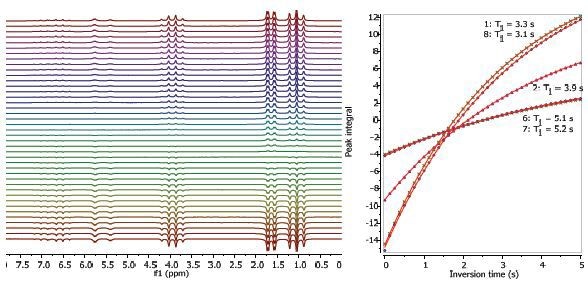
Figure 2. Proton T1 relaxation time measurement of 25% ethyl crotonate in CDCl3.
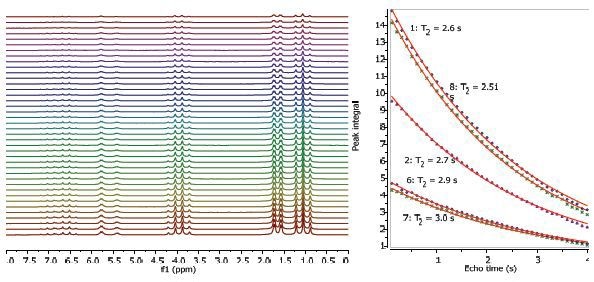
Figure 3. Proton T2 relaxation time measurement of 25% ethyl crotonate in CDCl3.
2D COSY
Figure 4 shows the 2D COSY spectrum in which two spin systems (6,7,8) and (1,2) can be clearly seen. For instance, the methyl group at the 8 position bonds to the 2 CH groups at 6 and 7 positions, while the methyl group at the 1 position bonds to the ethylene group at the 2 position. No coupling occurs at positions (6,7,8) to either 1 or 2.
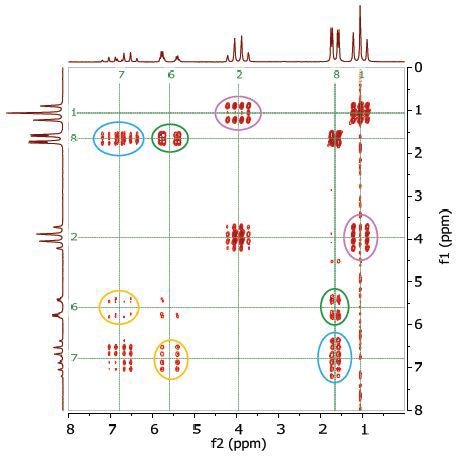
Figure 4. COSY spectrum of 25% ethyl crotonate in CDCl3. The cross-peaks and corresponding exchanging protons are labeled by colour-coded arrows and ellipses.
2D Homonuclear J-Resolved Spectroscopy
The chemical shift in the 2D homonuclear j-resolved spectrum appears along the f2 or direct dimension, and the effects of coupling between protons appear along the f1 or indirect dimension. This enables the entire assignment of chemical shifts of multiplets, and thus can help in measuring unresolved couplings. Additionally, a decoupled 1D proton spectrum is produced by the projection along the f1 dimension. The 2D homonuclear j-resolved spectrum of ethyl crotonate together with the 1D proton spectrum (blue line) is shown in Figure 5.
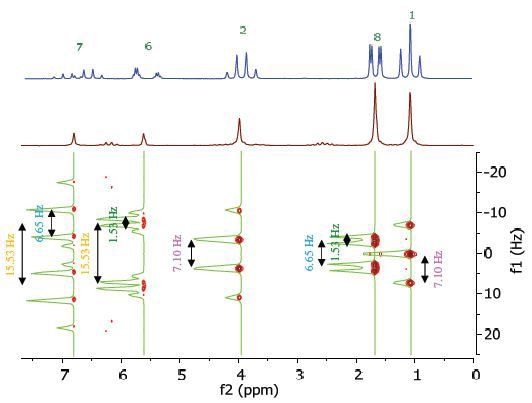
Figure 5. Homonuclear j-resolved spectrum of 25% ethyl crotonate in CDCl3. The multiplet splitting frequencies for different couplings are colour-coded as in Figure 4.
The projection which is vertical reveals how the overlapping multiplets disintegrate into a single peak, making the 1D spectrum more simplified. Peak multiplicities are produced by vertical traces from peaks in the 2D spectrum, as indicated by green lines in Figure 5, and help in determining the frequencies of proton-proton coupling.
When coupling frequencies are compared between different peaks, data can be obtained regarding which peaks are bonded to each other. Moreover, information regarding the coupling strength can be obtained from the size of the coupling frequency. These couplings substantiate the results of the COSY experiment shown in Figure 4.
However, in this experiment, the effects of second order coupling appear as additional peaks in the f1 dimension that are equidistant from the coupling partners suitably detached from the zero frequency in the f1 direction. These peaks offer direct proof of second order coupling partners, but are generally ignored as artefacts. Figure 6 shows these coupling partners and additional peaks are labeled by arrows and ellipses that are colour-coded.
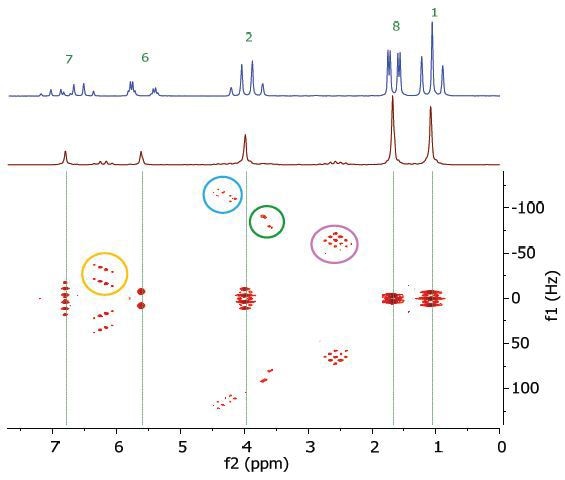
Figure 6. Homonuclear j-resolved spectrum of 25% ethyl crotonate in CDCl3, displaying the additional peaks due to strong couplings.
1D 13C Spectra
Figure 7 shows the 13C NMR spectra of 25% ethyl crotonate in CDCl3. Since the 1D Carbon method is highly susceptible to the 13C nuclei in the specimen, it clearly and easily 9 resonances. The 13C DEPT method utilizes polarisation transfer between carbon and proton nuclei. In this experiment, only carbons coupled to protons are seen.
Given the fact that the DEPT spectra do not display the peak at 167ppm, they are assumed to be part of the quaternary carbons. The DEPT-135 and the DEPT-45 experiments render signals of CH3, CH2 and CH groups, while the DEPT-90 experiment renders only the signal of CH groups. However, in the DEPT-135 experiment, the CH2 groups occur as negative peaks. This phenomenon is observed for the peak at 60ppm, which must belong to the carbon at the 2 position. When the three DEPT spectra are combined, the peak assignment illustrated in Figure 7 can be validated.
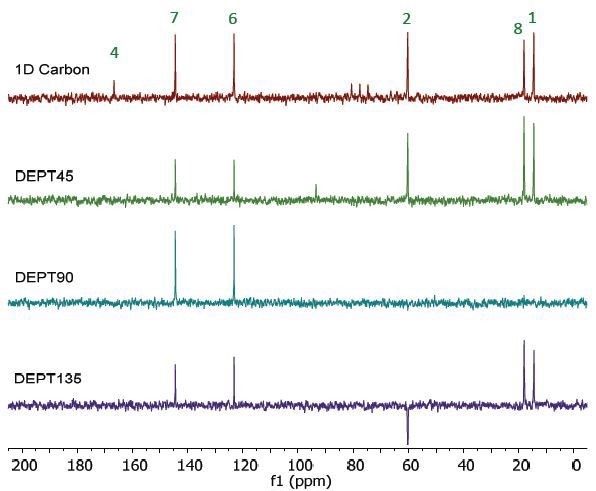
Figure 7. Carbon spectra of 25% ethyl crotonate in CDCl3.
Heteronuclear Correlation
A range of Heteronuclear Correlation (HETCOR) NMR experiments were developed to identify coupling partners of varied nuclei. The HETCOR experiment helps in detecting the proton signal that appears along the f1 dimension and the carbon signal along the f2 dimension. Figure 8 shows the HETCOR spectrum of 25% ethyl crotonate in CDCl3. in the 2D spectrum, the peaks reveal which proton is coupled to which carbon.
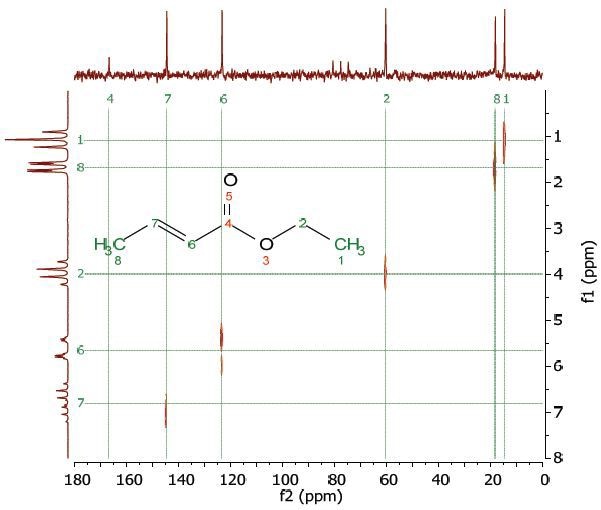
Figure 8. HETCOR spectrum of 25% ethyl crotonate in CDCl3.
Heteronuclear Multiple Quantum Coherence
Heteronuclear Multiple Quantum Coherence (HMQC) is similar to the HETCOR experiment and is utilized to associate proton resonances to the carbons that are coupled directly to those protons. But, in the HMQC experiment, the proton signal appears along the f2 dimension and the carbon signal along the f1 dimension. Figure 9 shows the HMQC spectrum of 25% ethyl crotonate in CDCl3. In the 2D spectrum, the peaks show which proton is coupled to which carbon.
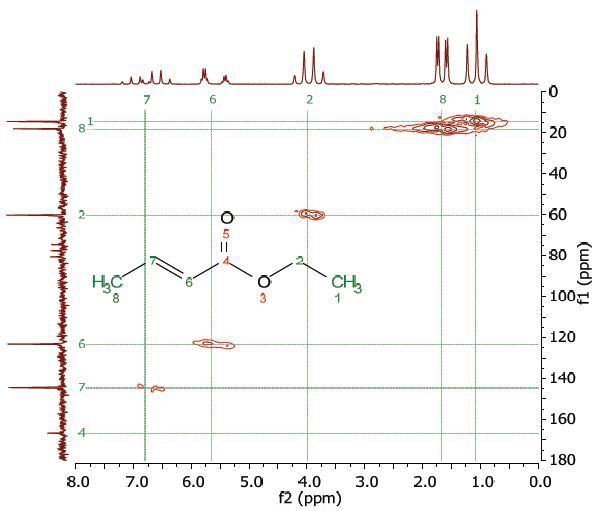
Figure 9. HMQC spectrum of 25% ethyl crotonate in CDCl3.
Heteronuclear Multiple Bond Correlation
The Heteronuclear Multiple Bond Correlation (HMBC) experiment can be utilized to achieve long-range correlations of proton and carbon via two or three couplings. Similar to the HMQC experiment, the proton signal appears along the f2 dimension and the carbon signal appears along the f1 dimension. Figure 10 shows the HMBC spectrum of 25% ethyl crotonate in CDCl3.
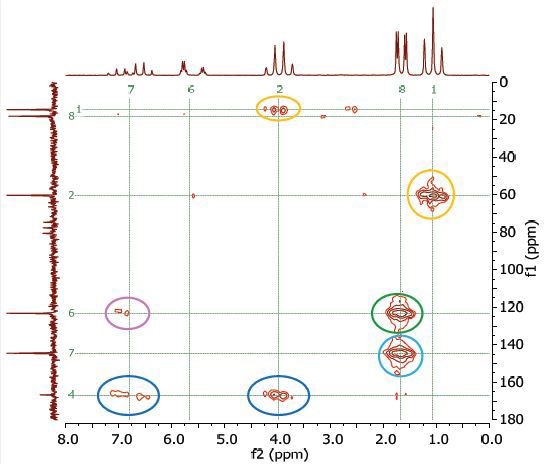
Figure 10. HMBC spectrum of 25% ethyl crotonate in CDCl3.
The couplings amid the molecular positions appear analogous to the couplings seen in the COSY spectrum; however, the HMBC experiment also displays couplings to quaternary carbons. These couplings are not seen either in HMQC or COSY experiment.
Magritek Spinsolve Carbon NMR Spectrometer
The Spinsolve Carbon (Figure 11) from Magritek ’s is a high-performance and low-cost benchtop NMR spectrometer that now has 1H, 19F and 13C capability, in addition to 2D experiments such as 2D JRES, COSY, HMQC, HMBC and HETCOR. This next-generation instrument is ideal for reaction monitoring, synthetic chemistry, chemistry education, and industrial QA and QC applications.

Figure 11. Spinsolve Carbon
Specifications of the Spinsolve Carbon are as follows:
- Resolution: 50% linewidth < 0.7 Hz (16ppb)
- Lineshape: 0.55% linewidth < 20Hz
- Frequency: 10.8MHz carbon, 42.5MHz proton
- Stray field: < 2 G all around system
- Magnet: Permanent and cryogen free
- Weight: 55kg
- Dimensions: 58 x 43 x 40cm
Conclusion
Magritek’s Spinsolve Carbon benchtop NMR spectrometer is a compact and low-cost instrument specifically designed for conducting common 2D experiments, such as 2D JRES, COSY, HMQC and HETCOR, amongst others. This revolutionary instrument provides excellent sensitivity and unparalleled performance and also saves time, space and cost.
About Magritek
Founded in 2004, Magritek is an advanced technology company exporting from Germany and New Zealand to customers all over the world. The initial technology and IP used in Magritek products was developed by research teams at RWTH Unviersity, Germany, and Massey University and Victoria University of Wellington in New Zealand.
Today, Magritek provides complete NMR and MRI system solutions for the oil and gas industry, as well as components and subsystems suitable for research laboratories and education.
This information has been sourced, reviewed and adapted from materials provided by Magritek.
For more information on this source, please visit Magritek.