Essential chemical intermediates in dye manufacture are nitroanilines. In this experiment series, p-nitroaniline is synthesized by a multi-step sequence as shown in Figure 1. This compound is used to synthesize the azo dye Para Red.
In order to prepare p- nitroaniline from acetophenone, by chemical reactions, one functional group on monosubstituted benzene is transformed into another and then an electrophilic aromatic substitution reaction is done to obtain the target compound. The various compounds prepared will be characterised by 1H NMR spectroscopy.
As shown in Equation 1, the first step for synthesizing p-nitroaniline is preparing acetophenone oxime from acetophenone. Oximes are highly crystalline featuring a carbon-nitrogen double bond with an OH group on the nitrogen atom. They are extensively used for the protection, purification and characterization of carbonyl compounds in synthetic organic chemistry. Oximes are versatile building blocks used to synthesize nitrogen containing compounds.
Figure 1. p-nitroaniline
Equation 1. Synthesis of acetophenone oxime.
Safety
Ethanol is flammable and must be handled with care. Acetophenone, sodium acetate trihydrate and acetophenone oxime are irritable to the skin, eyes and respiratory system.
Hydroxylamine hydrochloride is corrosive, avoid all contact and handle with care. Deuterochloroform is toxic and handle with care.
Synthesis of Acetophenone Oxime
Procedure
To a solution of 30mL water and 10mL ethanol solution in a 100mL round bottom flask,
acetophenone (3.75mL), hydrated sodium acetate crystals (7.50g) and hydroxylamine hydrochloride (3.75g) are added.
The mixture is heated by stirring on a hot water bath for 10min as shown in Figure 2. On the top of the solution, colorless oil drops should form. The mixture is cooled for 30min in an ice bath during which oil solidification must take place. If required, crystallization is induced by scratching the flask sides with a glass rod. The white solid is collected by filtration, washed with cold water and dried in the air. The crude product is recrystallized from boiling water ensuring that all oil droplets dissolved. The purified product is then filtered and dried and yield is recorded as shown in Figure 3.

Figure 2. Experimental setup for the synthesis of acetophenone oxime

Figure 3. Purified acetophenone oxime
Figure 4 shows the 1H NMR spectrum of acetophenone, a singlet (3H) at 2.60ppm, corresponding to the methyl group at position 1. The five aromatic protons at positions 4, 5 and 6 resonate as a multiplet between 7.39-8.09ppm.
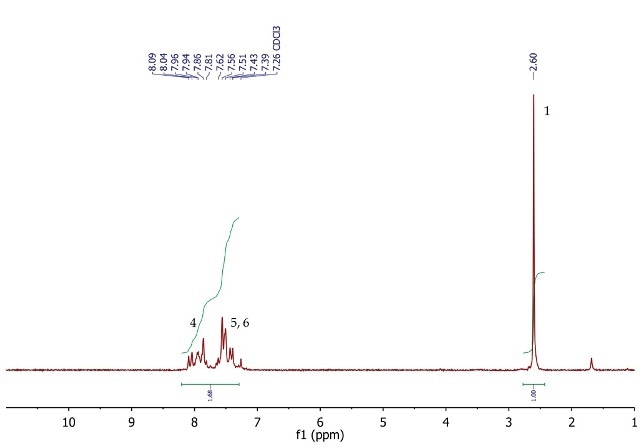
Figure 4. 1H NMR spectrum of acetophenone, CDCI3
Figure 5 shows the 1H NMR spectrum of acetophenone oxime wherein a singlet (3H) is seen at 2.36ppm, corresponding to the methyl group at position 1.
In the range 7.30 to 7.84ppm, the five aromatic protons are positions 4, 5 and 6 resonate as a broad multiplet. At 9.31ppm, the exchangeable NOH proton is observed as a broad singlet with a low peak integration value.
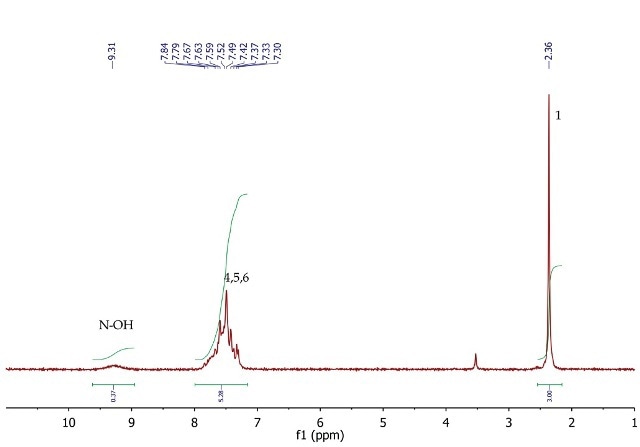
Figure 5. 1H NMR spectrum of acetophenone oxime, CDCI3
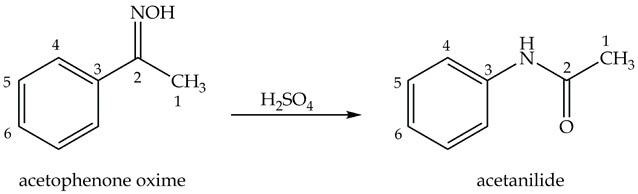
The next step in p-nitroaniline synthesis is preparing acetanilide from acetophenone oxime as shown in Equation 2.
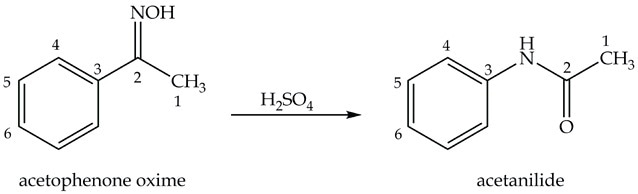
Equation 2. Synthesis of acetanilide.
Synthesis of Acetanilide: the Beckmann Rearrangement
Procedure
3mL concentrated sulfuric acid is placed in a boiling tube and heated in a hot water bath until the temperature of the acid reaches around 90 °C. Acetophenone oxime (3g) is added in small portions with stirring over a period of 20 min. For a further 15min the reaction mixture is stirred. The cool mixture is poured to crushed ice to precipitate the title compound. The solid is collected by filtration and washed with cold water. The crude product is recrystallized from 50mL water and yield is recorded.
The 1H NMR spectrum of acetanilide (Figure 6) shows a singlet (3H) at 2.14ppm, corresponding to the methyl group at position 1. The five aromatic protons at positions 4, 5 and 6 resonate as a broad multiplet between 7.02-7.65ppm. As seen in the peak integration The signal for the exchangeable NH proton may also be overlapping with the multiplet as suggested by the peak integration value.
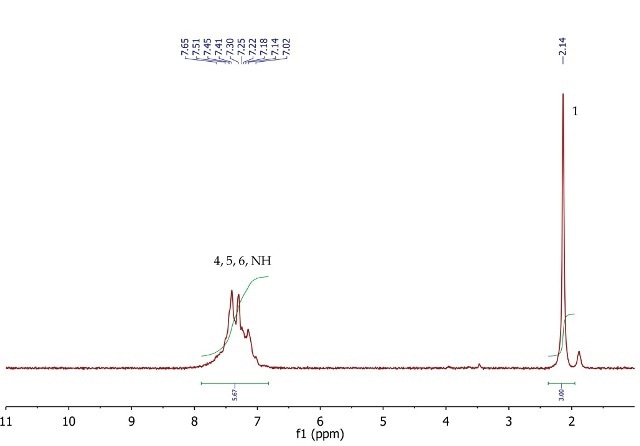
Figure 6. 1H NMR spectrum of acetanilide, CDCl3
The third step in synthesizing p-nitroaniline is nitration of acetanilide using a mixture of concentrated sulfuric and nitric acids to obtain nitroacetanilide (Equation 3). The main colorless product, p-nitroacetanilide, is almost insoluble in ethanol and filtering out is possible, while the yellow ortho isomer remains in the filtrate.
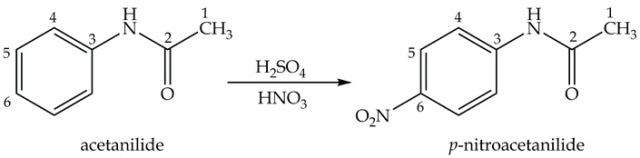
Equation 3. Synthesis of p-nitroacetanilide
Nitration of acetanilide
Procedure
1.5mL of glacial acetic acid is placed in a boiling tube and 1.5g of acetanilide is added. The mixture is stirred and 3mL of concentrated sulfuric acid is added. The hot reaction mixture is cooled in an ice/salt bath till there is a temperature drop to 0.5 °C.
Slowly, 0.6mL of fuming nitric acid is added ensuring the temperature does not rise above 20 °C. After adding, the reaction mixture is brought to room temperature and allowed to stand for 20 min. The mixture is poured onto ice and maintained for another 20min. The crude yellow solid is collected by filtration, washed with water and dried in the air. From the minimum amount of hot ethanol, p-nitroacetanilide is recrytsallized as a cream-coloured crystalline solid (Figure 7). Dry in the air and record your yield.
Scheme 3. Synthesis of p-nitroacetanilide.


Figure 7. (a)-(b). Crude and recrystallised p-nitroacetanilide.
The 1H NMR spectrum of acetanilide (Figure 8) shows a singlet (3H) at 2.05ppm, corresponding to the methyl group at position 1. The five aromatic protons at positions 4, 5 and 6 resonate as a broad multiplet between 6.97-7.74ppm. The exchangeable NH proton is also observed in DMSO-d6 at 9.91ppm as a broad singlet.
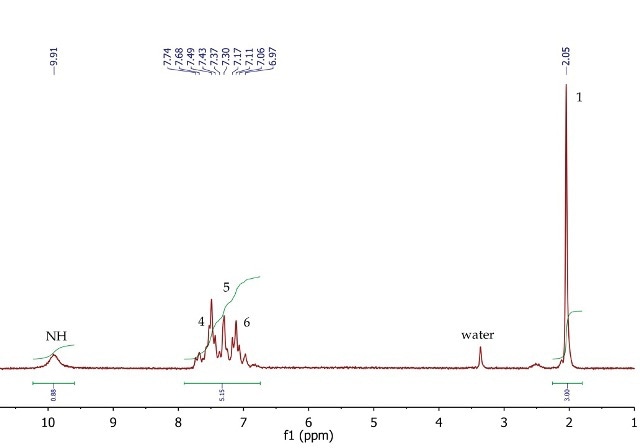
Figure 8. 1H NMR spectrum of acetanilide, DMSO-d6
p-nitroacetanilide’s 1H NMR spectrum (Figure 9) shows a singlet (3H) at 2.10ppm, corresponding to the methyl group at position 1. The four aromatic protons at positions 4 and 5 appear as a second order AA'BB' system, with two multiplets centred at 7.77 and 8.21ppm. The exchangeable NH proton is observed at 10.52ppm as a broad singlet.
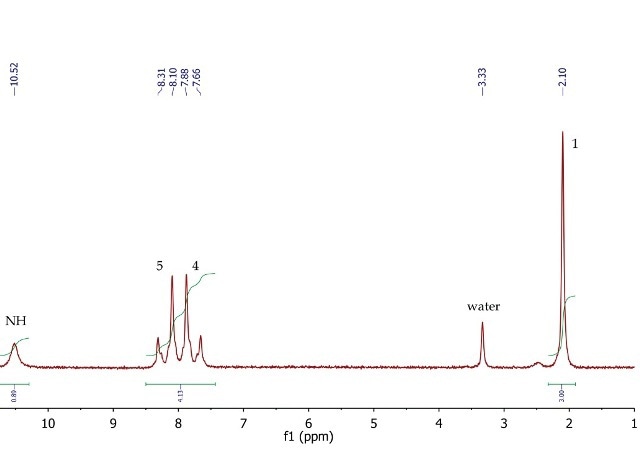
Figure 9. 1H NMR spectrum of p-nitroacetanilide, DMSO- cf6.
The hydrolysis of p- nitroacetanilide under acidic conditions is the final step in the synthesis of p-nitroaniline (Equation 4).
Hydrolysis of p-nitroacetaniline
Procedure
A 25mL round bottom flask is charged with a solution of concentrated sulfuric acid (4mL) and water (3mL). p-nitroacetanilide (0.7g) is added and heated the reaction mixture gently under reflux for 20min. The hot mixture is poured into cold water (20mL), and the pH of the solution is adjusted with sodium hydroxide solution (2M, approximately 120mL) until alkaline and a yellow precipitate is obtained. Cool the mixture in an ice bath. The crude yellow solid is collected by filtration , washed thoroughly with water and dried in the air. It is recrystallized from 1:1 ethanol/water mixture to obtain bright yellow crystals of the title compound.
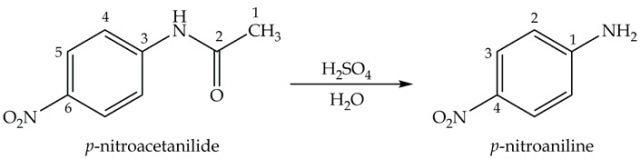
Equation 4. Synthesis of p-nitroacetanilide.
The 1H NMR spectrum of p-nitroacetanilide (Figure 10) shows two doublets at 6.59 and 7.95ppm, corresponding to the four aromatic protons at positions 2 and 3 respectively. The signal for the exchangeable NH2 protons is overlapping with the doublet at 6.59ppm, as indicated by the 2:1 peak integration values.
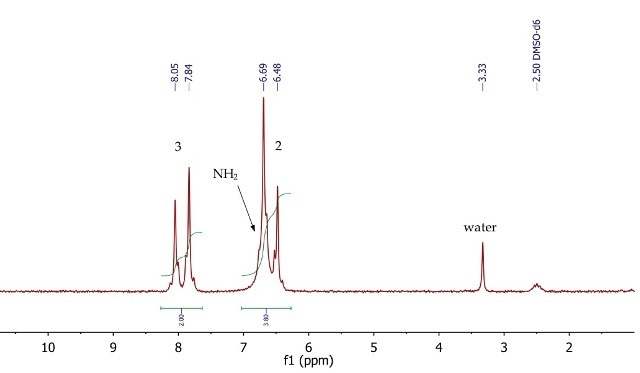
Figure 10. 1H NMR spectrum of p-nitroanilide, DMSO-d6.
This information has been sourced, reviewed and adapted from materials provided by Magritek.
For more information on this source, please visit Magritek.