Lithium-ion batteries (LIB) possess a higher specific power and a higher specific energy compared to lead-acid batteries, making them the most popular source of power for portable telecommunication and computing equipment.
At present, the renewable energy market is exploring novel materials for stationary energy storage, the automotive industry involved in the development of portable batteries, and the electronics industry are the key drivers of battery research projects.
Generally, a battery consists of a pair of electrodes that are divided by a separator and immersed in the electrolyte to promote ion movement. The key component of the anode is usually graphite, while lithium transition metal oxides, such as LiMn2O4 (LMO) or Li(NixCoyMnz)O2 (NCM), are often used as active materials for the cathode. Composite electrodes with two or more active materials are also employed to attain certain cell properties.
The active materials are added with conductive agents such as carbon black, and binders such as polyvinylidenfluoride (PVDF) and subsequently applied to a metal foil (aluminum or copper), which is used as the current collectors. A mixture of LiPF6 is used as the electrolyte, for instance, ethylene carbonate (EC) and dimethyl carbonate (DMC) is commonly used.
The separator is polymeric and is very important. It usually contains polypropylene and polyethylene. The separator’s composition establishes the battery’s operating temperature and is important to ensure the safety of the battery.
Researchers aim to decrease the ageing process and increase the travel range, so cycle time, life time, and energy density have to be improved in addition to temperature-independent higher capacities and properties.
To reach this objective for commercial rechargeable batteries, studies are being carried out to enhance the numerous aspects of the cell design, including novel active materials for anodes and cathodes, like anode material integrated with silicon particles or nickel-rich or overlithiated NCM, high voltage spinels (e.g. LiNi0,5Mn1,5O4).
Analytical methods can be used to develop new electrode designs and active materials, as these methods can display numerous chemical and morphological properties in a short period of time.
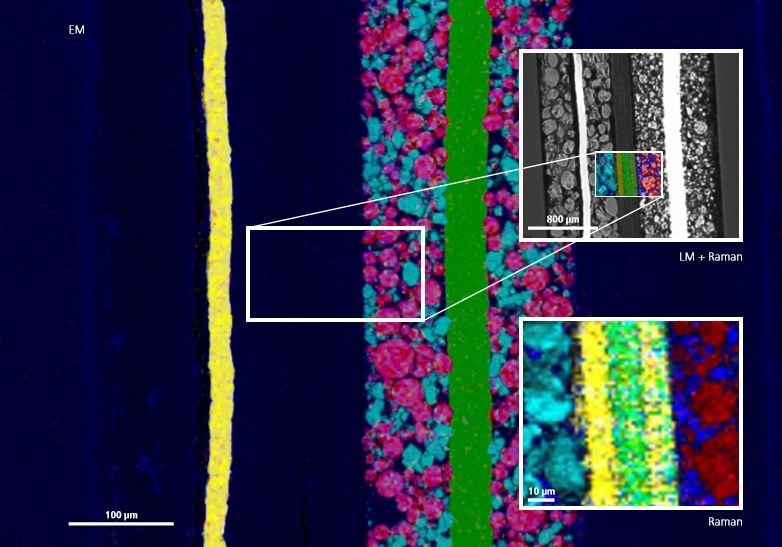
The in situ incorporation of a Raman system into a scanning electron microscope allows the analysis of the anode, cathode, and separator in a single measurement system, providing complementary data. Scanning electron microscope (SEM) provides a comprehensive analysis of the cathode material such as EDX measurements that show compositional data, while Raman imaging is perfect for studying binder, separator, and anode materials.
This article outlines a study involving a multi-modal method to investigate various components of a commercially available 18650 lithium ion battery in a single system. EDX measurements, combined with high resolution scanning electron microscope images revealed data regarding morphological changes, such as the actual composition of the cathode material and the cracking of particles subsequent to cycling.
In addition, Raman microscopy was employed to collect data regarding the polymeric separator and the carbon-containing anode material. The binder’s homogeneity was also made visible. A complete mapping or point measurement was performed, followed by superimposing the complementary data provided by Raman imaging and SEM.

This information has been sourced, reviewed and adapted from materials provided by Carl Zeiss Microscopy GmbH.
For more information on this source, please visit Carl Zeiss Microscopy GmbH.