Background Information
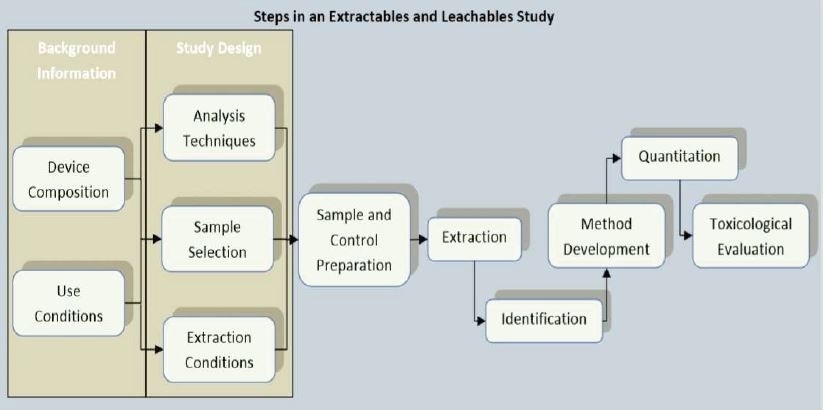
Materials of Construction
What additives are used?
Multilayer films?
Printing Inks?
Finished Packaging
Which surfaces does the product contact?
Are there interactions between components?
Is there opportunity for non-product contact components to migrate?
Are there any processing aids used?
Use Conditions
Extractables
Challenge the use condition
- Temperature
- Solvent Strength
- Time
Leachables
Mimic the most stringent use condition
Extraction Strategies
Cut and Cover Extraction

- Simple systems
- Irregularly shaped
- Articles requiring two-sided extraction
- Very large containers
Full Fill Extraction

- Bags
- Small containers
- Tubing
- Non-permeable
- Printed containers
One-Sided Extraction

Multi-layer
- Printed materials
- Coated materials
- Product contacts one side
Flow Through Extraction

Tubing
- Complex systems
- In-Line filters
- Connectors
Large Volume Dynamic Headspace

- Direct analysis of the entire article
- Very high sensitivity
- No risk of volatiles loss

 Please click here if you would like more information on the product in this article or a quote
Please click here if you would like more information on the product in this article or a quote
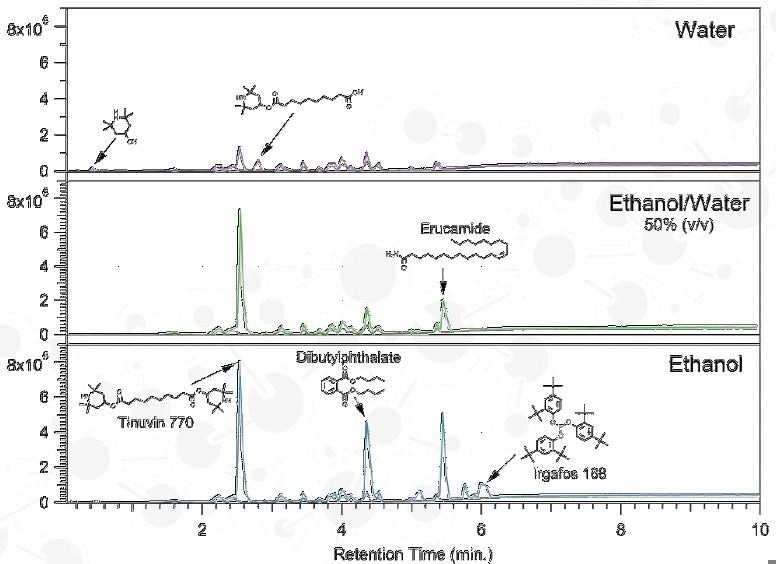
Extraction Solvent
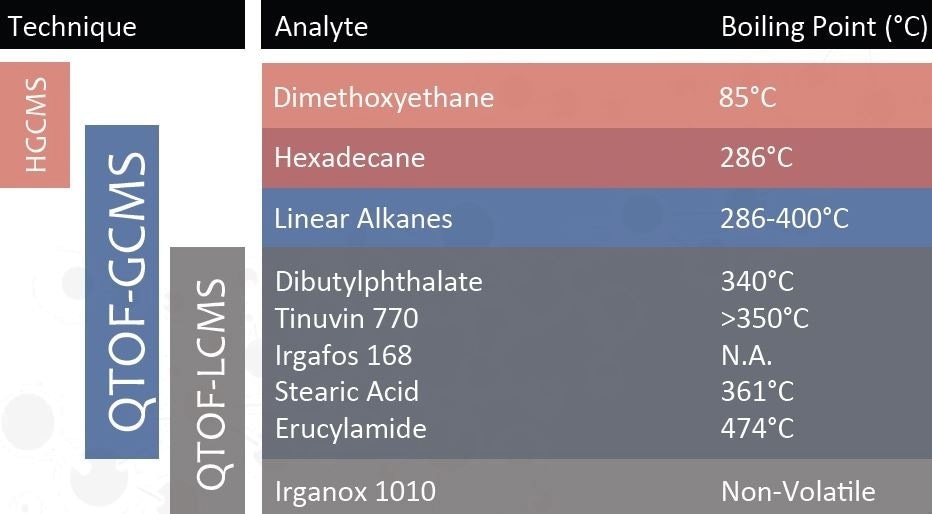
From the background information provided for a container closure system the probable extractables were:
| Expected Extractable |
Type |
Log P |
Boiling point |
| Erucamide |
Hydrophobic |
8.8 |
474 °C |
| Linear Alkanes |
Hydrophobic |
8.859 |
250-400 °C |
| Dibutylphthalate |
Hydrophobic |
4.72 |
340 °C |
| Dimethoxyethane |
Volatile |
-0.2 |
85 °C |
| Irganox 1010 |
Non-volatile |
23 |
N/A |
| Irgafos 168 |
Non-volatile |
15.5 |
N/A |
| Tinuvin 770 |
Basic |
6.3 |
N/A |
| Stearic acid |
Acidic |
8.23 |
361 °C |
| Sodium benzenesulfonate |
Anionic |
N/A |
N/A |
Effect of Solvent Polarity
Agilent 1290 Infinity UHPLC; Agilent 6520 QTOF-MS
Column: Agilent Zorbax Eclipse Plus C8; 1.8 µm, 2.1x50 mm
Electrospray IonizaAon (ESI); Polarity: positive
Extract Preparation

Example:
- 250 mL per bag
- Worst case 3 bags per day
- Product contact surface area 310 cm2
- Safety concern threshold (SCT) = 0.15 µg/day
AET (µg/bag) = 0.15 µg/day/3 bags/day = 0.05 µg/bag
Extract Vol. (mL) = 310 cm↑2/6 cm↑2/mL =52 mL
LOD = 0.05 µg/bag / 52 mL/bag ≈ 1 ng/mL
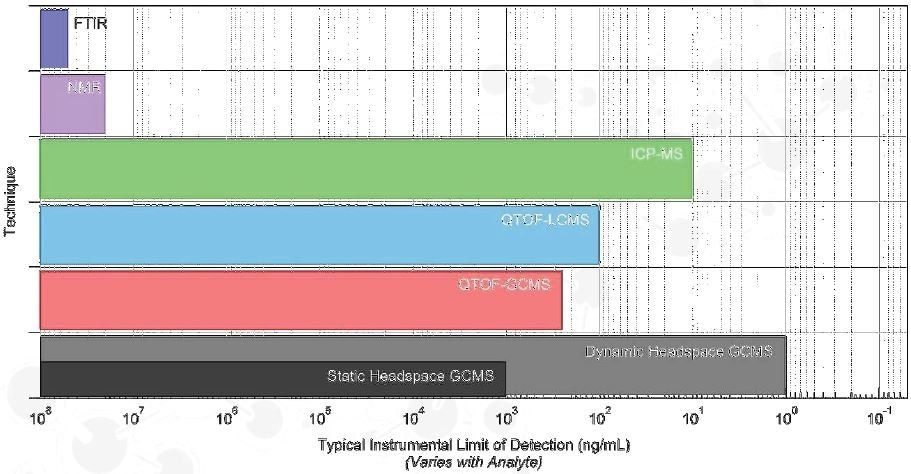
Qualitative Analysis
Concentration of Extracts
Extractables and Leachables can be lost during concentration
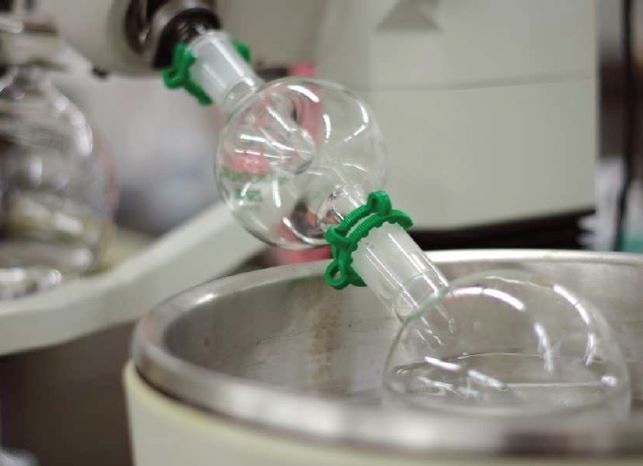
Loss depends on
- Volatility of the analyte(s)
- Extract handling
Best Practices
- Mild conditions
- Method validation
- Headspace analysis
Qualitative Analysis
No universal analytical technique exists for E&L analysis
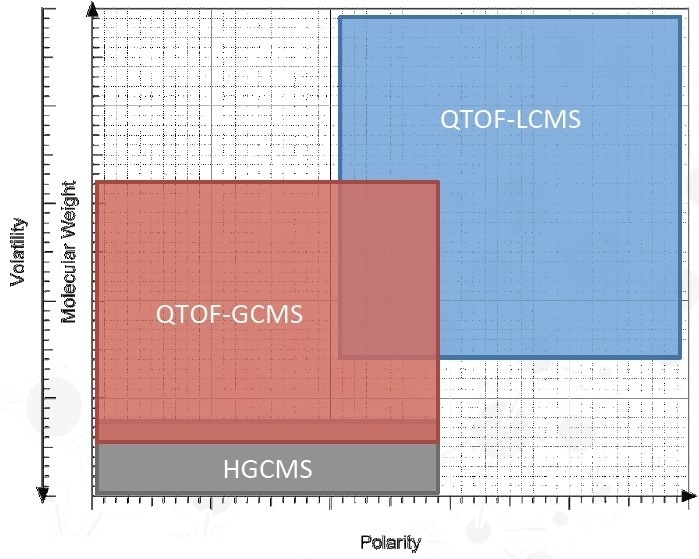
- Headspace GCMS
- QTOF-GCMS
- Volatiles
- Semi-Volatiles
- Non-polar analytes
- QTOF-LCMS
- ICP-MS
Extraction Conditions
Ethanol Extract
| Expected Extractables |
Type |
Log P |
Boiling point |
| Erucamide |
Hydrophobic |
8.8 |
474 °C |
| Linear Alkanes |
Hydrophobic |
8.859 |
250-400 °C |
| Dibutylphthalate |
Hydrophobic |
4.72 |
340 °C |
| Dimethoxyethane |
Volatile |
-0.2 |
85 °C |
| Irganox 1010 |
Non-volatile |
23 |
N/A |
| Irgafos 168 |
Non-volatile |
15.5 |
N/A |
| Tinuvin 770 |
Basic |
6.3 |
N/A |
| Stearic acid |
Acidic |
8.23 |
361 °C |
| Sodium benzenesulfonate |
Anionic |
N/A |
N/A |
Qualitative Analysis
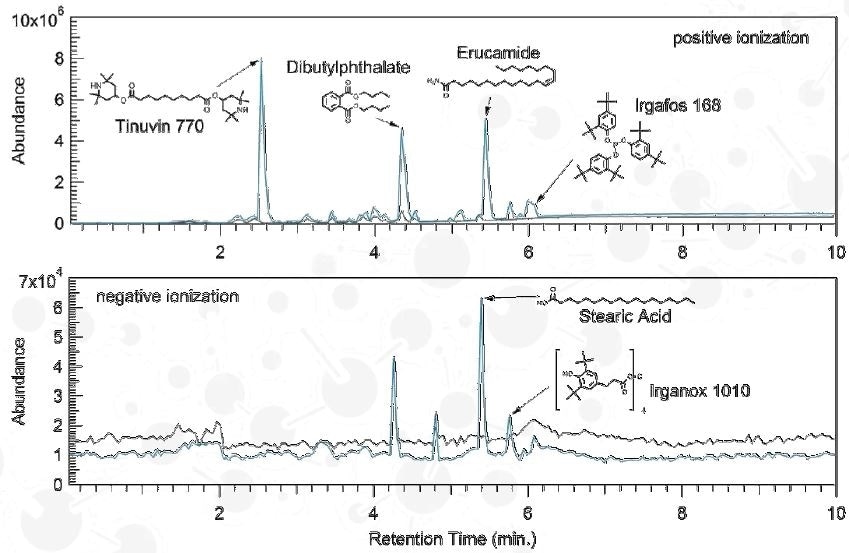
Agilent 1290 Infinity UHPLC; Agilent 6520 QTOF-MS
Column: Agilent Zorbax Eclipse Plus C8; 1.8 µm, 2.1x50 mm
Electrospray Ionization (ESI)
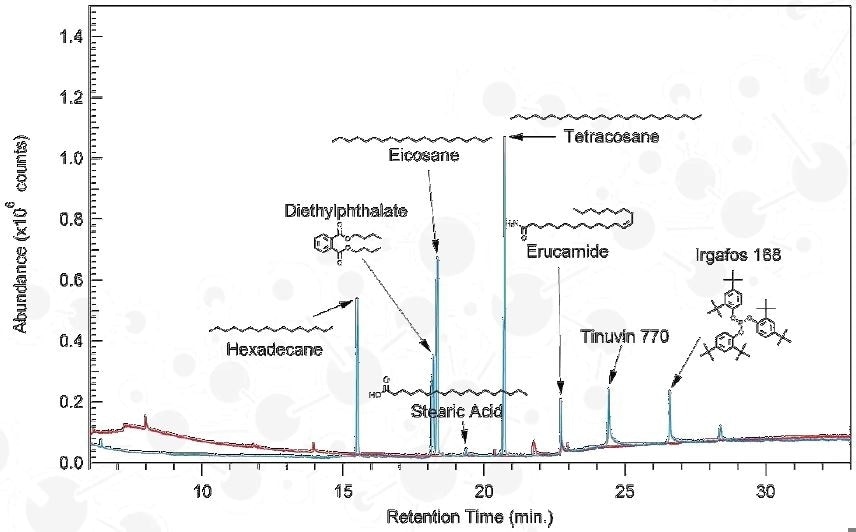
Agilent 7890B GC; Agilent 7200 QTOF-MS
Column: DB-5MS UI; 0.25 mm x 30 m, 0.25 µm
Electron Impact Ionization
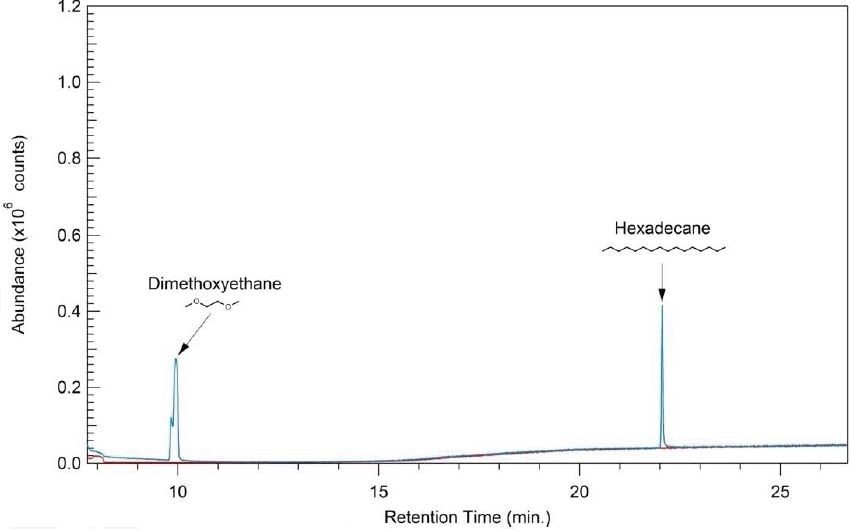
Agilent 6890 GC; Agilent 5973 inert MSD
Electron Impact Ionization
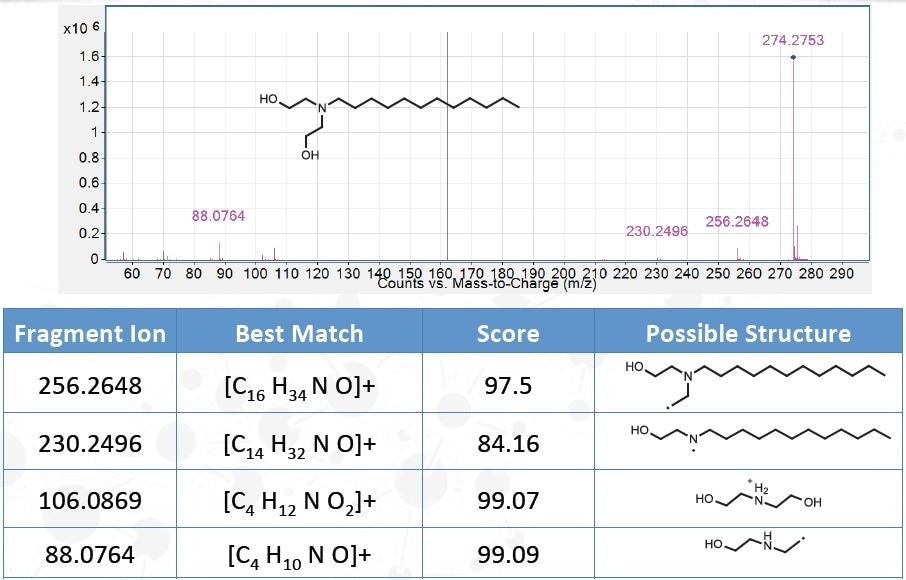
Identification of Unknowns

Database Searches
- Commercial Databases (NIST, Wiley)
- Jordi Proprietary Additive and Oligomer Databases
QTOF-GC/QTOF-LC
- Molecular Formula Generation (MFG)
- MS/MS for QTOF -LCMS
- CI for QTOF-GCMS
| m/z |
Best Match |
Species |
Mass |
Score (MFG) |
Diff. (ppm) |
DBE |
| 274.2731 |
C16 H35 N O2 |
[M+H]+ |
273.2660 |
94.66 |
2.73 |
0 |
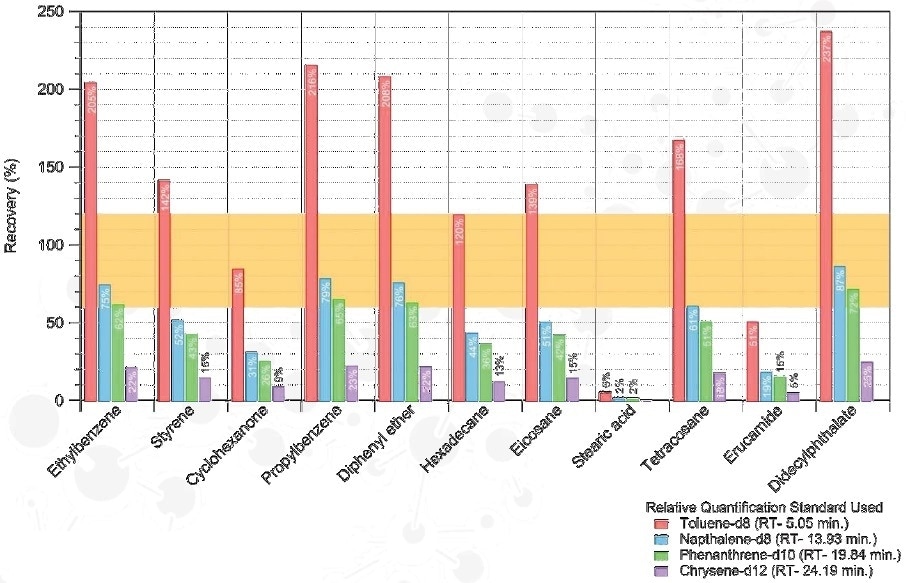
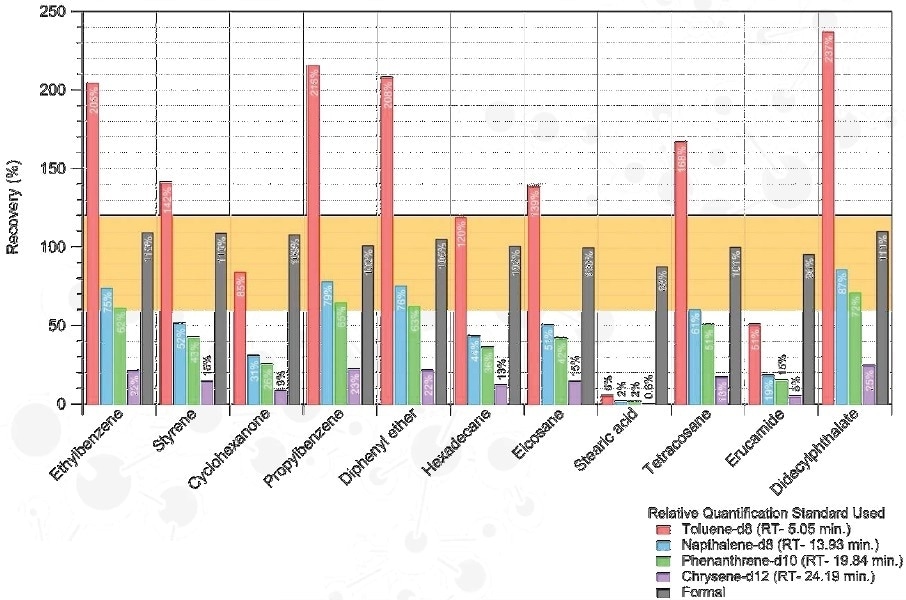
Quantitative Strategies
Relative Quantitation
- Quantification of compounds observed is carried out against surrogate standards
- Accuracy is based on the surrogate standard used
Estimated Analyte Conc. = Observed Analyte Peak Area / Surrogate Peak Area x Surrogate Conc.
Formal Quantitation
Confirmed compounds are quantified against an analytical standard at a series of concentrations
Methodologies:
- External Standardization
- Standard Addition
- Needs high purity analytical standards
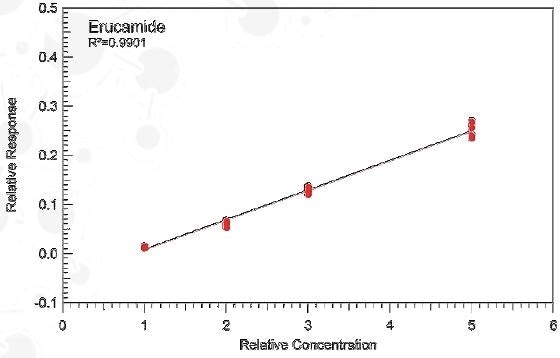
Quantitative Method Development

Quantitative Methods:
- Dynamic Headspace GCMS
- UHPLC-UV
- UHPLC-CAD
- QTOF-GCMS
In formal quantitation limit of quantitation (LOQ) can be improved using:
- Targeted MS/MS
- Large Volume Injection
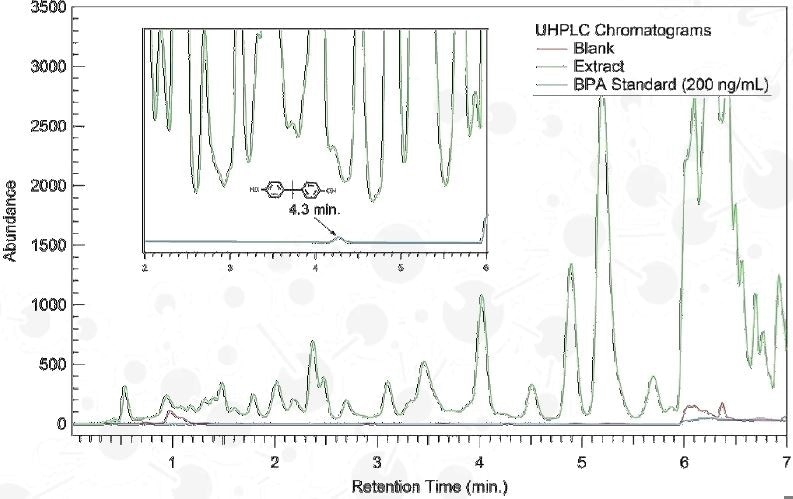
Agilent 1290 Infinity UHPLC
Column: Zorbax SB-C18, 1.8 µm, 100 x 2.1 mm
Mobile Phase: H2O – ACN Grad.
Detection: 230 nm (DAD)
2D UHPLC
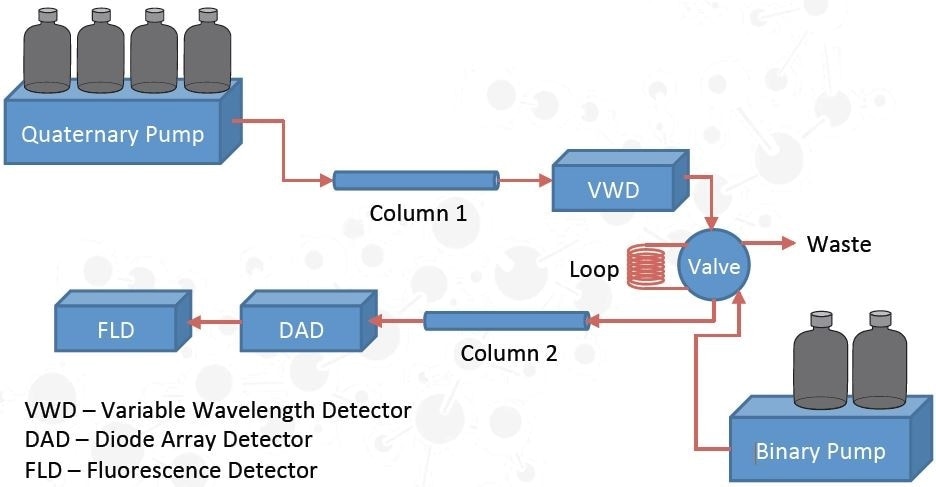
Quantitative Method Development
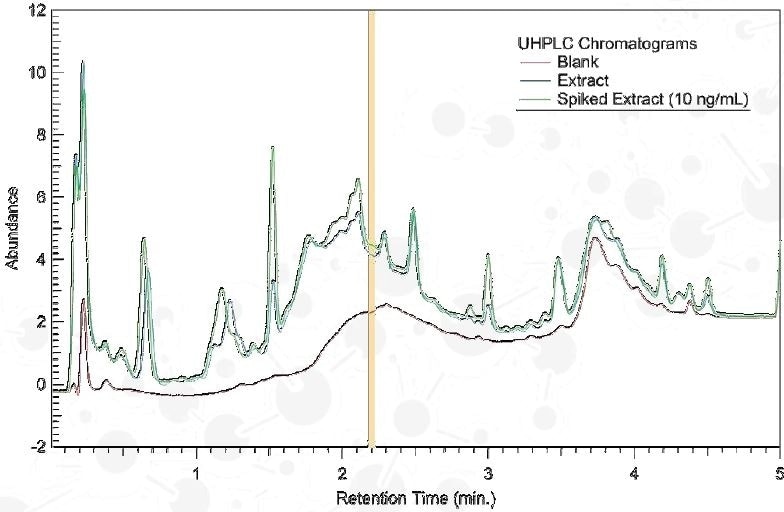
Column: Zorbax SB-C18; 1.8 µm, 2.1x50 mm
Mobile Phase: H2O – ACN Gradient
Detection: 230 nm (VWD)
Collection Mode: Heart-cutting; 2.18-2.22 minutes (40 µL)
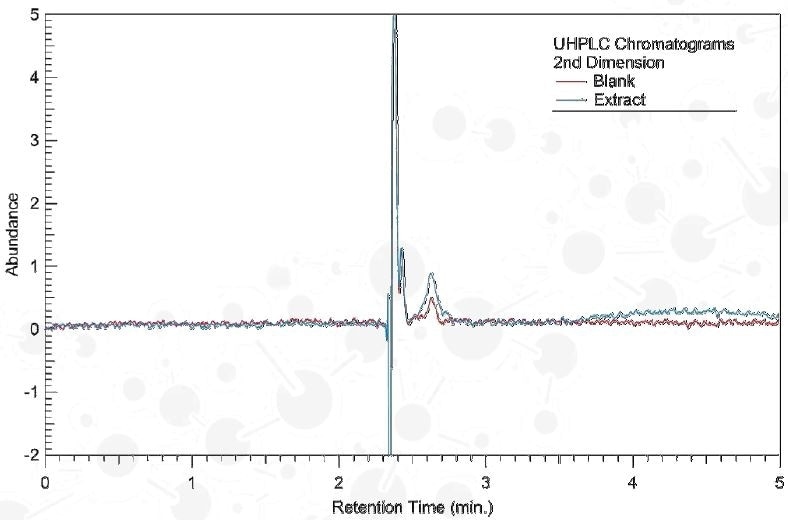
Column: Eclipse Plus Phenyl-Hexyl; 1.8 µm, 2.1x50 mm
Mobile Phase: H2O – ACN Isocratic
Detection: 230 nm (DAD)
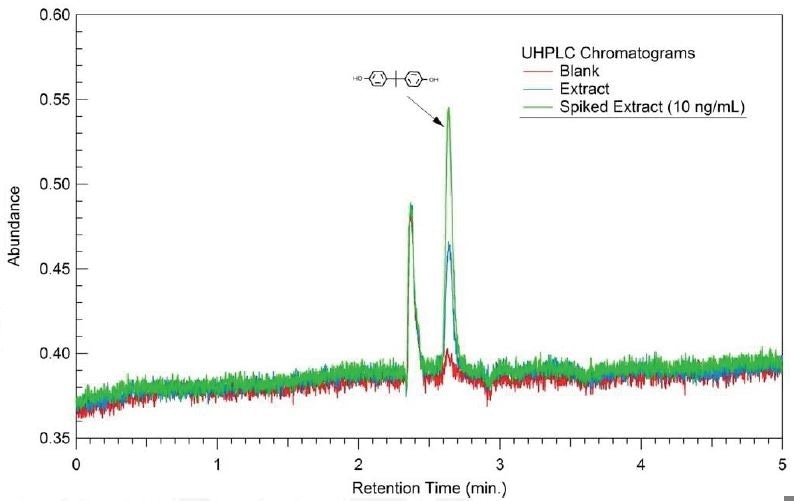
Column: Eclipse Plus Phenyl-Hexyl; 1.8 µm, 2.1x50 mm
Mobile Phase: H2O – ACN Isocratic
Detection: Fluorescence; 225 nm excitation, 310 nm emission
A successful study has:
- Definitive identifications
- Accurate quantification
- Appropriate extraction conditions
- Careful concentration of extracts

This information has been sourced, reviewed and adapted from materials provided by Jordi Labs.
For more information on this source, please visit Jordi Labs.