In undergraduate organic chemistry courses, nucleophilic substitution reactions are often carried out as an experiment. Reactions that occur at saturated carbons are chiefly categorized as SN1 or SN2, where S stands for substitution, N for nucleophilic, and the number denotes the molecularity of the reaction—1 for a unimolecular process and 2 for a bimolecular process.
Nucleophilic Substitution Through SN1 or SN2 Mechanism
In the SN2 reaction (Figure 1), the attack of the nucleophile and removal of the leaving group take place at the same time in a combined process and its rate is proportional to the concentration of both the nucleophile and the alkyl halide. However, in the SN1 reaction, there are two separate steps: initially, gradual dissociation of the leaving group to produce a carbocation intermediate, and then rapid attack of the nucleophile to form a new bond. As the first step is rate-determining, the rate is based only on the alkyl halide concentration. Various factors such as the structure of the alkyl halide, the nucleophile, the leaving group, and the solvent decide whether the nucleophilic substitution progresses through the SN1 or SN2 mechanism and also the rate of the reaction.
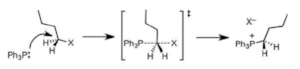
Figure 1. Mechanism of an SN2 reaction
Experiments for analyzing both the mechanisms are carried out in the organic chemistry course of an undergraduate program. Those reactions involve the use of qualitative observations to analyze the two mechanisms. For example, a solution of NaI in acetone is often added to various alkyl halides to investigate the factors that have an impact on the SN2 reaction. The rate of the reactions is ascertained by the formation of NaX, which precipitates out of solution (Figure 2). In general, this results in discrepancies between students since the formation of the precipitate cannot be easily quantified and is largely dependent on the person observing the experiment. The following questions often come across the mind of a teaching assistant in an organic lab during every term: Is this a precipitate or just the solution turning cloudy? Is the reaction done? and so on.
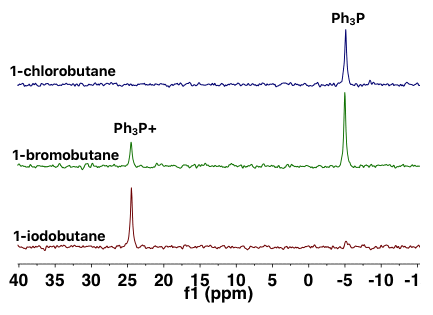
Figure 2. Test tubes containing several alkyl halides after the addition of NaI in acetone.
Using NMReady for Acquiring Data Related to Nucleophilic Substitution
The NMReady enables acquiring quantitative data related to a nucleophilic substitution reaction. This article highlights one such experiment. One of the most significant factors governing the mechanism of the reaction is the leaving group in the alkyl halide. In order to evaluate that effect, 1-chlorobutane, 1-bromobutane, and 1-iodobutane were used as electrophiles and triphenylphosphine was used as the nucleophile. Figure 3 illustrates the stacked spectra and the ratio between the phosphonium product and the unreacted starting material.

Figure 3. 31P{1H} NMR spectra of a series of reactions between alkyl halides and triphenylphosphine.
The results demonstrate that alkyl halides with larger, more polarizable halides are highly reactive substrates in SN2 reactions with 1-chlorobutane and 1-iodobutane at the extreme ends of reactivity. The first one does not exhibit any reaction, whereas the second one typically realized a 100% yield of the phosphonium salt product.
Conclusion
In this article, the role of the most significant factors determining the rate of an SN2 reaction has been investigated in detail.

This information has been sourced, reviewed and adapted from materials provided by Nanalysis Corp.
For more information on this source, please visit Nanalysis Corp.