Scanning Electron Microscopes (SEM) are powerful magnification tools that utilize focused beams of electrons to obtain detailed topographical, morphological and compositional data. In the past samples have been limited to solid, inorganic samples small enough to fit inside a chamber under moderate vacuum pressure. In this article, we will look at equipment and techniques that increase the utility of modern SEM to provide biologists with more insight into cell function.
There are a wide variety of ways to image biological tissue and cells in SEM, the aim is to image the biological material in as close to its natural state as possible without introducing any artefacts by any treatments we apply to it. However, the material does not tolerate vacuum very well, gives a low signal, tends to charge and is soft, if it needs to be cut.
Taking SEM to the Next Level
A variety of techniques are available to image the outside of biological tissues or cells and provide biologists with more insight into cell surface structure. Here we will discuss some of the approaches available:
- Negative Stain
- Fix, dehydrate, critical point dry, metal coat
- Use variable pressure and a cold stage
- Cryo – rapid freeze, etch, coat
Each of these techniques utilizes different detectors, microscope conditions and gives us different views of the sample, to better understand external structure.
Negative Staining
Negative staining is a simple approach designed to visualize structures such as bacteria, viruses, and isolated tissue cells. It relies on staining around the sample, as in the diagram below. A bacteria or virus suspension is placed on a transmission electron microscope (TEM) grid, dried and then stained with a heavy metal stain such as PTA (Phosphotungstic acid) or Uranyl Acetate to introduce contrast to the background.
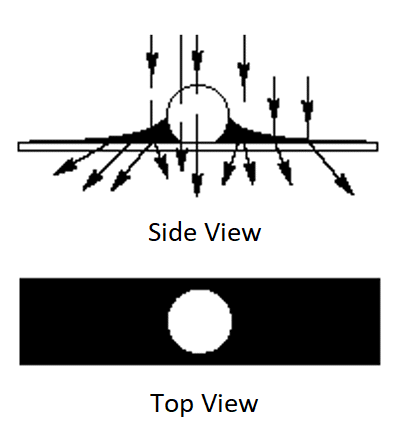
The grid is placed in the multigrid STEM holder and then imaged using the STEM detector. The retractable STEM detector is inserted below the sample allowing bright field, dark field and high angle dark field imaging.
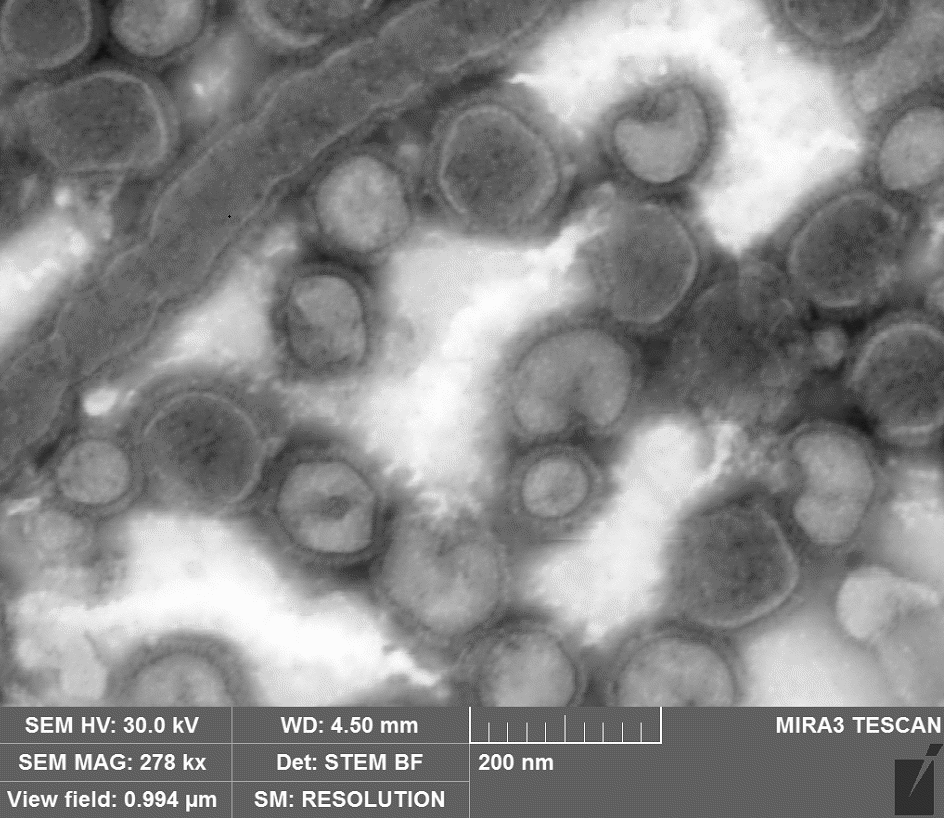
Negatively stained Influenza Virus, usually spherical or ovoid in shape, 80 to 150 nm in diameter, with glycoprotein spikes, imaged using the STEM detector
Fixing, Critical Point Drying, and Coating
Fixing, critical point drying, and coating is also a well-known technique. Here the sample is fixed with glutaraldehyde which cross links proteins before a secondary fixative, osmium tetroxide. Osmium Tetroxide stabilizes cell and organelle membrane lipids.
Osmium is also a heavy metal which helps greatly with contrast in an organic low atomic number material. This is followed by dehydration with alcohol or acetone and then the samples are critically point dried before being coated with a metal such as gold.
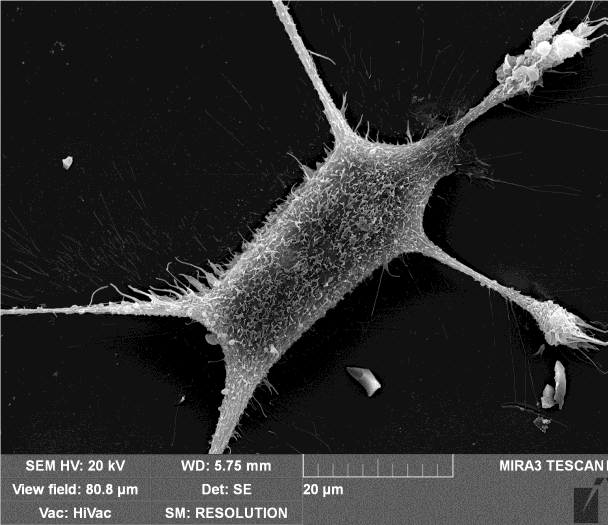
Mouse Embryonic Fibroblasts
Variable Pressure and Cold Stage
A less used approach is imaging samples using variable pressure and a cold stage. Variable pressure or low vacuum techniques allow non-conductive samples such as tissue and cells to be visualized successfully.
Any hydrated sample in a vacuum will dry out eventually. By degrading the vacuum, the rate at which water is removed can be reduced. When this is combined with a cold stage it is possible, by working at the triple point, to image water in the SEM. One consideration is that while the sample is not exposed to any chemical treatments, the vacuum of the SEM can introduce artefacts. Also, the poorer the vacuum the weaker the signal, so long scan times are often required.
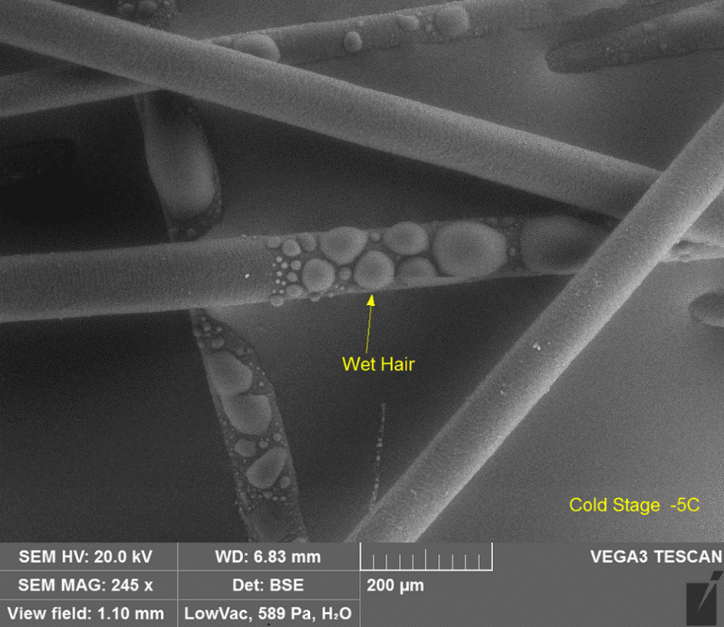
Hair with water droplets imaged at close to 600 Pa with the cold stage slightly below freezing
Cryo, Rapid Freeze, and Coat
In this approach the cells are rapidly frozen to keep them in as close to their natural state as possible. The rate of freezing is critical to avoid ice crystal damage to the cells, the faster the freezing the smaller the ice crystals.
Rapid freezing can be achieved in a range of methods including liquid nitrogen plunge freezing or high pressure freezing. Once the cells are frozen, they can be coated with metal like gold, to make them conductive and to help with signal.
The cells have to be maintained in a frozen state throughout the entire process of coating and imaging. This requires a cryo stage in the SEM chamber and a transfer device to get the sample from the rapid freezing device into the SEM.
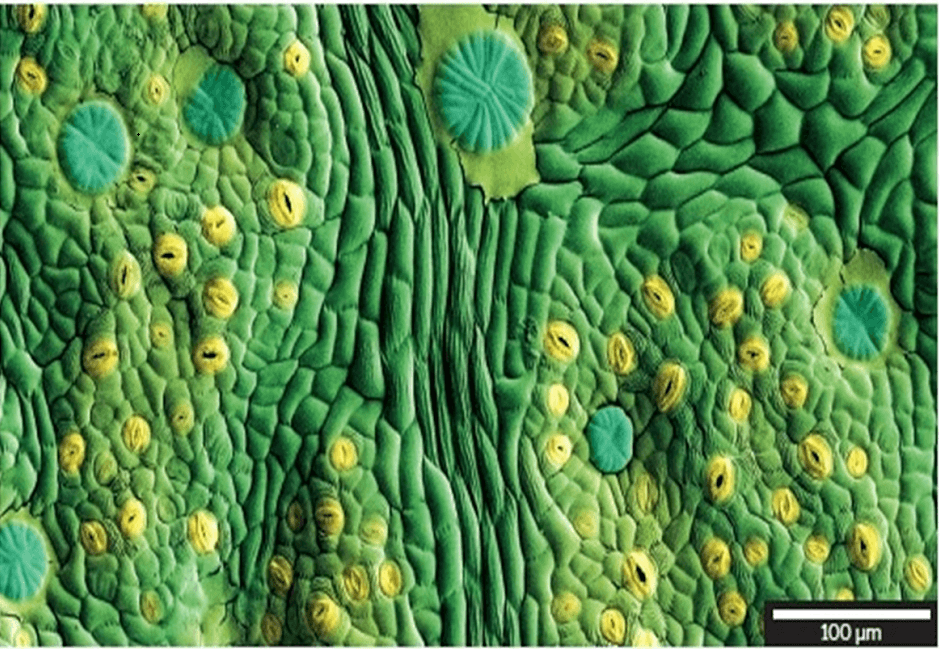
CRYO prepared ash leaf (artificially coloured)
STEM Detector for Single Cells
With STEM detectors the electron beam is focused to a fine spot typically 0.05 - 0.2 nm, which is scanned over the sample in a raster system with the beam parallel to the optical axis. This enables the electron probe to be focused to allow images with sub-Angström resolution.
The advantage of STEM imaging in biological samples is the high-contrast of annular dark-field images, which can allow imaging of biological samples without the need for staining. STEM has been widely used to solve a number of structural problems in molecular biology.
The VEGA 3
The VEGA3 is a versatile thermionic emission SEM system intended for both high and low-vacuum operations designed to accomplish a wide range of SEM applications. The extended low-vacuum mode allows imaging of non-conductive samples and is ideal for biological specimens particularly when paired with the cold stage.
The system also has a range of chamber sizes for different sample sizes, a five axis motorized stage and many ports for future development. The VEGA3 can also be equipped for cryo-operation and transfer.
The MIRA 3
The MIRA3 configurations include a range of chamber sizes with ideal geometry for both low- and high-vacuum operations. It can be also configured with a large AMU chamber allowing SEM analysis of extraordinary large samples. MIRA3’s chamber configurations extend the analytical capabilities of the system, providing the ability to perform fine observations of the sample surface even for extra-large specimens.
This instrument is also equipped with an LE-BSE detector, which is optimized to increase detection sensitivity at low energies. This system can also be equipped with a retractable STEM detector, enabling bright field, dark field and high angle dark field imaging, and a cryo stage and transfer system.
TESCAN Group
Founded in 1991 by a group of managers and engineers from Tesla with its electron microscopy history starting in the 1950’s, today TESCAN is a globally renowned supplier of Focused Ion Beam workstations, Scanning Electron Microscopes and Optical Microscopes. TESCAN’s innovative solutions and collaborative nature with its customers have won it a leading position in the world of nano- and microtechnology. The company is proud to participate in premier research projects with prominent institutions across a range of scientific fields. TESCAN provides its clients with leading-class products in terms of value, quality and reliability. TESCAN Group is the North American arm of TESCAN Group, a multinational company established by the merger of Czech company TESCAN, a leading global supplier of SEMs and Focused Ion Beam workstations, and the French company ORSAY PHYSICS, a world leader in customized Focused Ion Beam and Electron Beam technology.

This information has been sourced, reviewed and adapted from materials provided by TESCAN Group.
For more information on this source, please visit TESCAN Group.