The lifespan of lithium-ion batteries (LIBs) and the related capacity fade are primarily influenced by the degradation of electrodes due to the deactivation of active materials. Studying this degradation is a challenging task that requires appropriate methods for performing micro- and nanoscale analysis.
A scanning electron microscope equipped with a focused ion beam (FIB-SEM), uniquely combined with a compact time-of-flight secondary ion mass spectrometer (ToF-SIMS),1 enables the identification of LIB capacity fade mechanisms. This identification is based on the loss of lithium inventory caused by parasitic chemical reactions or lithium trapping within electrode particles.2,3
FIB-SEM with the ToF-SIMS method also makes it possible to identify the chemical composition of contaminants within the battery material, as well as giving researchers the power to study of the degradation process of electrode particles, amongst other key parameters.
The FIB-SEM combined with the ToF-SIMS technique also allows for the chemical identification of contaminants within the battery material and the study of the degradation process of electrode particles.
Furthermore, the ability to correlate SEM observations with ToF-SIMS and other analytical techniques, such as energy dispersive X-ray spectroscopy (EDS) and Raman spectroscopy, within the same FIB-SEM system, extends analytical capabilities.4 This integration provides a comprehensive understanding of LIB material behavior from mechanical, chemical, and electrochemical perspectives.
Study of Parasitic Chemical Reactions – Comparative Analysis of Pristine and Cycled Electrode Material
Samples (pristine/cycled) based on NMC cathode and graphite anode preparation:
- Slurry derived from commercial chemical substances: electrode particles, PVDF binder, and carbon black,
- Slurry applied to a metal foil (Al/Cu) and subsequently dried,
- Battery assembly and electrolyte filling, cycling (1000x), and disassembly (cycled samples only).
Analysis:
- Cross-section parameters – tilted orientation, 100 μm wide, >60 μm deep,
- ToF-SIMS: 30 keV Xe+ primary ions, current 220 pA, area 30/40 x 30/40 μm2 for cathode/anode.

Image Credit: TESCAN Group
![The ToF-SIMS positive and negative ion mode mass spectra from analysis of NMC battery electrodes prepared in a moisture environment resulting in PVDF binder dehydrofluorination and generating chemically stable lithium fluoride at the interface between PVDF and cathode particles [2]](https://www.azom.com/images/Article_Images/ImageForArticle_23655_17165427453326830.png)
The ToF-SIMS positive and negative ion mode mass spectra from analysis of NMC battery electrodes prepared in a moisture environment resulting in PVDF binder dehydrofluorination and generating chemically stable lithium fluoride at the interface between PVDF and cathode particles 2. Image Credit: TESCAN Group
![The SEM image of the analyzed cross-section area and ToF-SIMS maps showing the position of PVDF binder dehydrofluorination (see fluorine map) [2]](https://www.azom.com/images/Article_Images/ImageForArticle_23655_17165427821891864.png)
The SEM image of the analyzed cross-section area and ToF-SIMS maps showing the position of PVDF binder dehydrofluorination (see fluorine map) 2. Image Credit: TESCAN Group
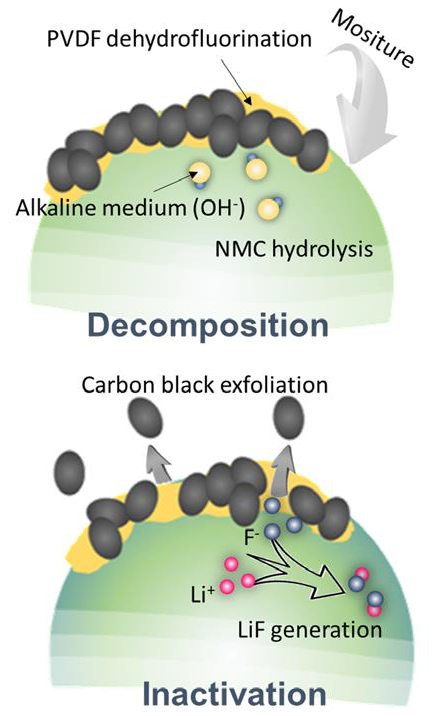
Schematic of the parasitic chemical reaction process. Image Credit: TESCAN Group
Electrode Particles Degradation Evaluation
![ToF-SIMS distribution maps of the representative transition-metal-oxide and transition-metal-fluorine showing local degradation of NMC particle by the parasitic chemical reaction of Mn with fluorine-based chemical. The particle degradation is very well visible by SEM imaging. NMC cathodes are identical to the samples mentioned above [2]](https://www.azom.com/images/Article_Images/ImageForArticle_23655_17165428093951800.png)
ToF-SIMS distribution maps of the representative transition-metal-oxide and transition-metal-fluorine showing local degradation of NMC particle by the parasitic chemical reaction of Mn with fluorine-based chemical. The particle degradation is very well visible by SEM imaging. NMC cathodes are identical to the samples mentioned above 2. Image Credit: TESCAN Group
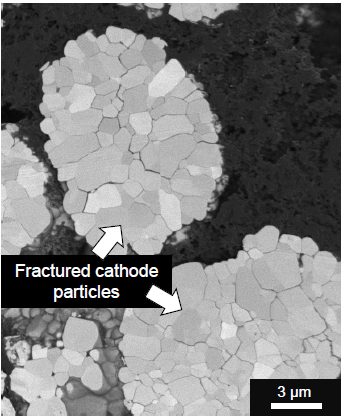
Example of the SEM observation of NMC particle damage in the form of fracturing as a result of the cycling process. Image Credit: TESCAN Group
![The correlation of Raman spectroscopy data with SEM images of NMC cathode particles before and after the cycling process (analysis in the cross section) with evident a change in the phase of the particles after the cycling and the presence of a particle not participating fully in the cycling [4]](https://www.azom.com/images/Article_Images/ImageForArticle_23655_17165428419646469.png)
The correlation of Raman spectroscopy data with SEM images of NMC cathode particles before and after the cycling process (analysis in the cross section) with evident a change in the phase of the particles after the cycling and the presence of a particle not participating fully in the cycling 4. Image Credit: TESCAN Group
Loss of Lithium Inventory Investigation
![In a properly working battery in a fully charged state, lithium in the form of ions left the cathode and moved to the anode side. The ToF-SIMS method allows the identification of the battery capacity fade origin based on the loss of lithium inventory in the form of trapped lithium within NMC cathode particles (analysis in the cross-section) [3]](https://www.azom.com/images/Article_Images/ImageForArticle_23655_17165428619557605.png)
In a properly working battery in a fully charged state, lithium in the form of ions left the cathode and moved to the anode side. The ToF-SIMS method allows the identification of the battery capacity fade origin based on the loss of lithium inventory in the form of trapped lithium within NMC cathode particles (analysis in the cross-section) 3. Image Credit: TESCAN Group
Instrumentation
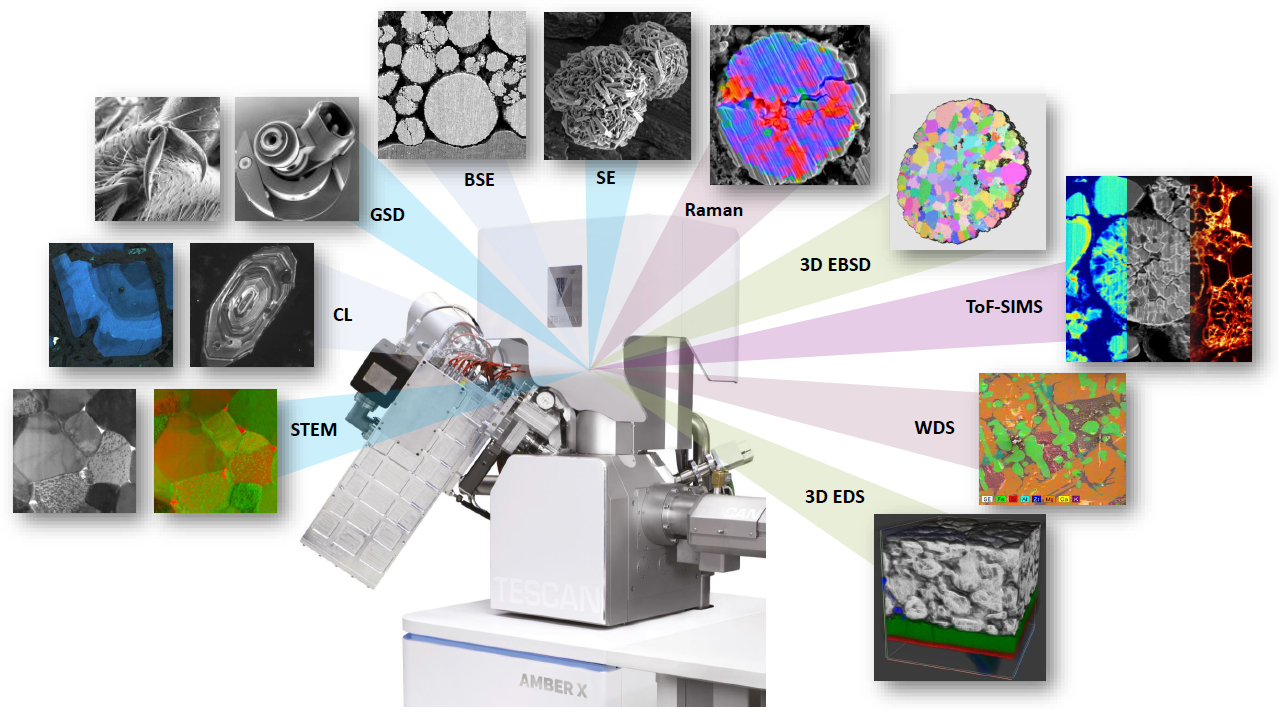
Analytical potential of TESCAN AMBER X plasma FIB-SEM. Image Credit: TESCAN Group
Conclusion
- NMC lithium-ion battery degradation was studied with considerable success in the FIB-SEM system fitted with the ToF-SIMS and Raman spectroscope. The same plasma Xe FIB was used for cross-section preparation and subsequent high-spatial resolution ToF-SIMS analysis.
- The decomposition of the PVDF binder by hydrolysis of the cathode material during cathode electrode preparation releases fluoride radicals. These radicals form stable lithium fluoride, leading to a loss of lithium inventory.
- Manganese, with a greater dominance than cobalt and nickel, leads to a formation of inactive fluoride deposition on the cathode side.
- The residual “trapped” Li in the cathode of the fully charged battery can be identified using ToF-SIMS.
- New opportunities are created using subsurface analysis based on SEM observation, ToF-SIMS, EDS, Raman spectroscopy, etc., creating a deeper and more comprehensive understanding of inner battery processes.
References
- J.A. Whitby, et. al., Advances in Mat. Sci. and Eng., 2012, 1-13, (2012)
- X. Yao, et. al., Energy Environ. Mater., 5, 662-669, (2021)
- T. Sui, et. al., Nano Energy, 17, 254-260, (2015)
- D.J. Miller, et. al., Microsc. Microanal., 25, 862-863, (2019)

This information has been sourced, reviewed and adapted from materials provided by TESCAN Group.
For more information on this source, please visit TESCAN Group.