Using the Brookhaven Instruments NanoBrook Omni and BI-ZTU autotitrator, the automated quantification of particle size as a function of pH is rapid and straightforward. This article exhibits the efficacy of these instruments to quantify the aggregation of Iron Oxide nanopowder showing a transformation in pH.
Academic and industrial processes that utilize iron oxide nanoparticles are wide-ranging. Of the nanomaterials, iron oxide has contemporarily aroused considerable interest from researchers as a result of its low toxicity, stability, and magnetic features.1 Due to the significant potential for processes utilizing iron oxide, an increased understanding of the impact of chemical changes to the surface of the nanoparticle is of particular benefit. This study aims to establish the onset of aggregation as a result of a change in pH.
Aggregation can happen as a result of changes in pH or temperature. When nanoparticles coalesce, they appear bigger, as demonstrated in Figure 1. The hydrodynamic diameter, often known as the effective diameter, is the diameter of a sphere that diffuses at the same rate as the particle under measurement.
The hydrodynamic diameter depends not only on a particle core’s size but also on the ionic strength of the medium. Outer layers, including surfactants on the particle’s surface, will also alter a particle’s apparent size. The apparent size of the particle will also adjust with any changes to the particle surface affecting the diffusion rate.
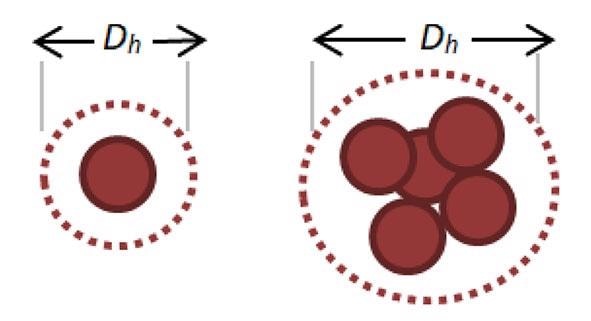
Figure 1. Representative increase in Hydrodynamic Diameter as Nanoparticles Aggregate.
Materials and Methods
A suspension was produced by mixing 5.8 mg of iron oxide nanopowder Fe3O4 from Skyspring Nanomaterials with 20 mL of filtered MilliQ water. A 0.2 μm filter was utilized to strain the MilliQ water three times. The suspension was subsequently sonicated in a Branson 2800 low energy sonic bath for five minutes. A Brookhaven Instruments BI-ZTU Autotitrator was loaded with four reagents: Fluka Analytical 0.1 M nitric acid; 1 mM nitric acid; 0.1M potassium hydroxide; and 1 mM potassium hydroxide.
The suspension was examined in a range spanning a pH of 2 to 12, increasing in 2 pH unit steps. The solution was investigated five times at each pH, for 5 minutes per run, utilizing dynamic light scattering (DLS). Measurements were taken utilizing Brookhaven Instruments NanoBrook Omni Particle Size Analyzer and BI-ZTU Autotitrator, and were examined with Particle Solutions, version 3.5 software.
The effective diameter of iron oxide nanoparticles was established from pH 2 to 12 in 2 pH unit steps, in a suspension of MilliQ water. Iron oxide nanoparticle aggregates in suspension were analyzed by plotting the measurements gathered over the ranges of tested pHs.
Results and Discussion
Dynamic light scattering (DLS) method can identify the effective diameter or size of a nanoparticle, while an auto titrator can assist in automating the process so that changes to particles’ apparent size can be identified as a function of pH. Table 1 shows that the mean effective diameter of iron oxide nanoparticles, on an intensity weighted basis, in acidic solution, occurred in the size range of 1023 to 1029 nm. The effective diameter of iron oxide nanoparticles on an intensity weighted basis, in basic solution, occurred in the size range of 4258 to 4520 nm.
Table 1. Representative Effective Diameter with Change of pH
| Effective Diameter with Change of pH |
| pH |
Effective Diameter |
| 2 |
1029 nm ± 102 |
| 4 |
1023 nm ± 143 |
| 6 |
1157 nm ± 200 |
| 8 |
4258 nm ± 1463 |
| 10 |
3243 nm ± 740 |
| 12 |
4520 nm ± 2316 |
Table 2 demonstrates that the mean polydispersity of iron oxide nanoparticles was 0.329 to 0.361 in acidic solution. The polydispersity was 0.315 to 0.334 in basic solution. Clearly, there is a reasonably constant transformation in the width of the distribution.
Table 2. Representative Polydispersity with Change of pH
| Polydispersity with Change of pH |
| pH |
Polydispersity |
| 2 |
0.329 ± 0.044 |
| 4 |
0.361 ± 0.035 |
| 6 |
0.329 ± 0.043 |
| 8 |
0.315 ± 0.055 |
| 10 |
0.297 ± 0.069 |
| 12 |
0.334 ± 0.107 |
Figure 2 exhibits the results of all quantifications of the prepared suspension at differing pH values. This implies that, as the pH value grows, the effective diameter increases and the stability decreases. The stability decreases as the particles near the isoelectric point. The onset of aggregation is apparent for iron oxide between pH 4 and 8, the same region where the zeta potential approaches zero.
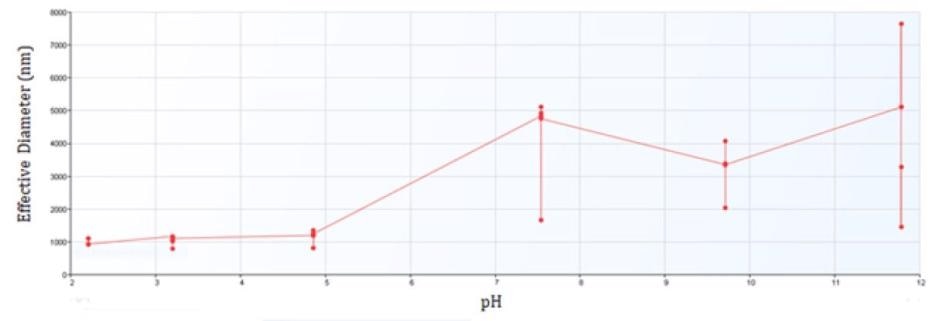
Figure 2. Effective Diameter of Iron Oxide from pH 2 to 12.
Figure 3 demonstrates that, as the pH multiples, the effective diameter of the particles increases.
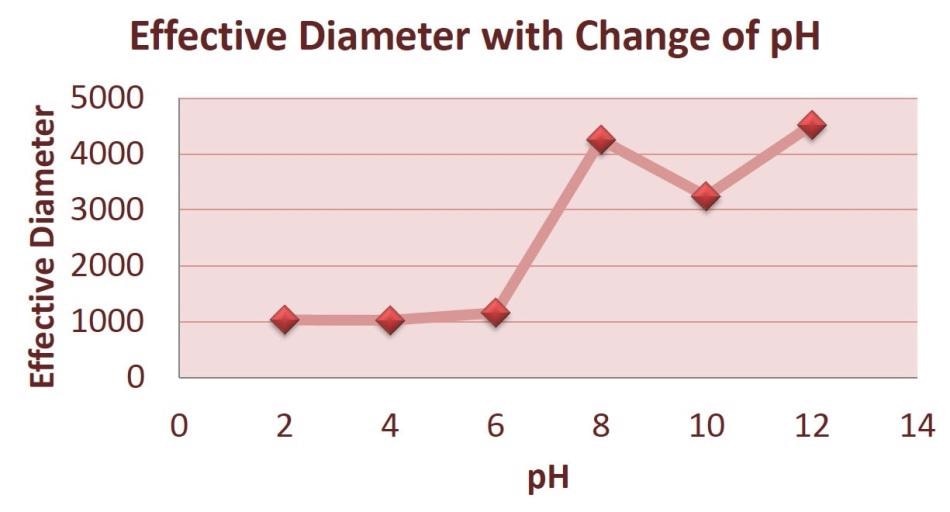
Figure 3. Average Effective Diameter with Change of pH.
The isoelectric point for iron oxide is at pH 7.5. This is the range where particle aggregation takes place as a result of low charge on the particle surface. Quantification of zeta potential with a change of pH provides additional support to particle aggregation at the same pH range and can be undertaken to utilize the same Brookhaven Instruments Omni and BI-ZTU.
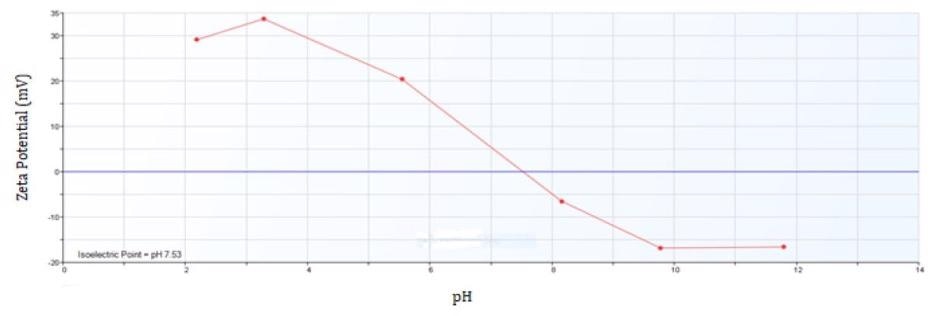
Figure 4. Zeta Potential of Iron Oxide from pH 2 to 12.
The multimodal size distribution displays multiple particle populations in the sample. The iron oxide nanopowder embodied three particle size populations. The particles shifted in size during aggregation, and with three populations remaining from pH 2 to pH 12.
As demonstrated in Figure 5, the multimodal size distribution exhibited in the upper graph is at pH 2 and the multimodal size distribution in the lower graph is at pH 12.
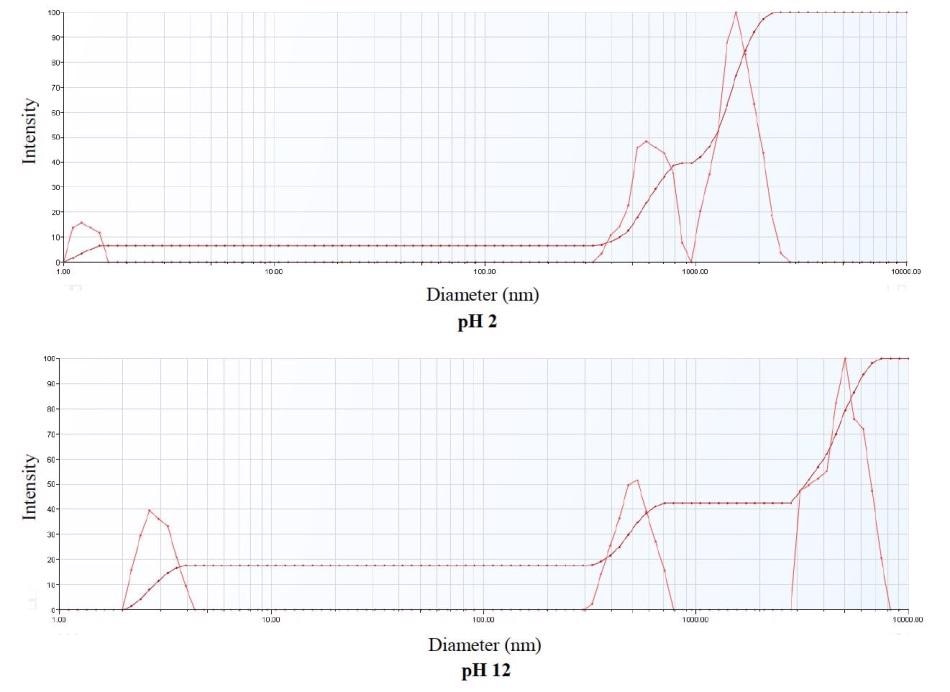
Figure 5. Multimodal Size Distribution Comparison at pH 2 and pH 12.
Conclusion
As a result of their functionality and efficiency, the Brookhaven Instruments NanoBrook Omni Particle Size Analyzer and auto titrator are effective tools to identify the onset of aggregation of iron oxide nanoparticles. This general methodology is applicable to multiple categories of nanomaterials and is customizable to specific applications. The incorporation of different variables, including time dependence or additive titrations like salts or dispersing agents, is also possible. Numerous industries can benefit from the functionality and efficiency of the NanoBrook Omni alongside the ZTU.
References and Further Reading
- Wu, Wei, et al. “Designed Synthesis and Surface Engineering Strategies of Magnetic Iron Oxide Nanoparticles for Biomedical Applications.” Nanoscale, vol. 8, no. 47, 2016, pp. 19421–19474., doi:10.1039/c6nr07542h.

This information has been sourced, reviewed and adapted from materials provided by Brookhaven Instrument Corporation.
For more information on this source, please visit Brookhaven Instrument Corporation.