Nuclear magnetic resonance (NMR) spectroscopy is considered as a useful analytical method. This is because the data obtained from an NMR spectrum complements the data achieved from other types of molecular spectroscopy. In the majority of cases, the technique provides exclusive diagnostic data about the sample material.
When a high-concentration sample needs to be rapidly analyzed, benchtop NMR can perform well. This is shown by using the case of diethylfluoromalonate.
Diethylfluoromalonate is liquid at room temperature, and can be analyzed neat in a standard NMR tube
There are two frequency channels in the X-Pulse 60 MHz NMR spectrometer; one frequency channel acquires spectra of 19F and 1H, while the other obtains spectra of 13C. For optimal performance, the probe can be manually adjusted and matched. The first channel, in this case, was tuned to 1H and the pulse width was subsequently extended to ensure effective transmission of radiofrequency (RF) signals for the 19F experiment.

Method
A standard 5-mm NMR tube was filled with 0.4 mL of diethylfluoromalonate using a pipette and then placed in the X-Pulse 60 MHz spectrometer without any temperature preconditioning. The sample was subsequently run unlocked and the resultant data was processed using the Mnova software from Mestrelab.
Results
The results can be noticed in the scans given below. It must be noted that the spectra are approximately referenced. The 13C spectra were subjected to 1 Hz exponential apodization.
Achieving a higher number of scans offers the spectra with a more improved signal-to-noise ratio. This is specifically observed in low-sensitivity nuclei like 13C. Moreover, the quaternary carbon doublet (separated by the fluorine) signals at 166 ppm can be compared in Figures 1 and 2.
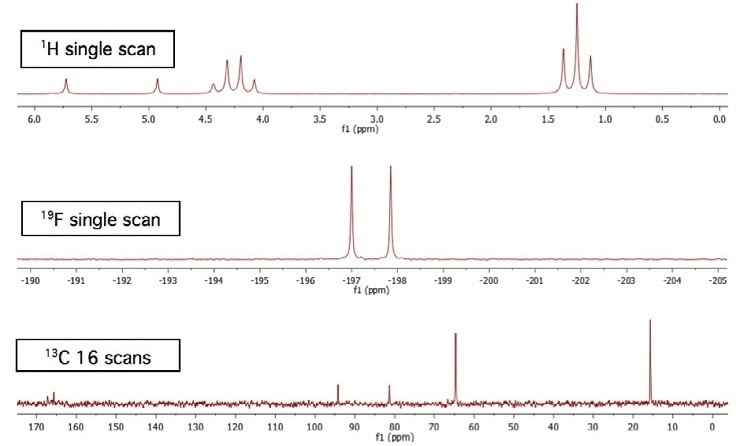
Figure 1. Total acquisition time for three spectra = approximately 75 seconds.
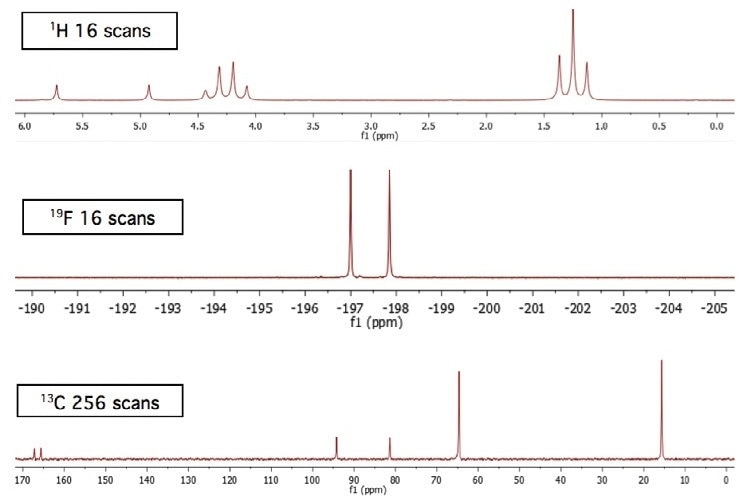
Figure 2. Total acquisition time for the three spectra is approximately 30 minutes.
Conclusion
This article shows how the benchtop NMR spectrometer can be used to obtain meaningful 1D spectra of different nuclei, from a sample of sufficiently high concentration.

This information has been sourced, reviewed and adapted from materials provided by Oxford Instruments Magnetic Resonance.
For more information on this source, please visit Oxford Instruments Magnetic Resonance.