
The advent of 2-dimensional (2D) NMR experiments is regarded as one of the biggest developments in NMR since its inception.
The introduction of such experiments has considerably boosted the scope of NMR in structural elucidation, and has also widened the complexity and range of issues that can be addressed.
These experiments consist of a series of 1-dimensional (1D) experiments that vary only through a time increment. This time increment is introduced via the pulse sequence, leading to a 2D array with two distinct time evolutions - the indirect measurement (t1) and the direct measurement (t2).
Subsequently, a 2D Discrete Fourier Transform of the data produces a 2D spectrum with frequency axes F1 and F2. At high field, 2D NMR experiments are routine, practical, and informative, and they now offer the same benefits for benchtop NMR.
Heteronuclear correlation experiments are an important class of 2D experiments. These experiments provide through-bond correlations between nuclei of two different elements, such as carbon and hydrogen, and are essential for establishing the structure of an unidentified molecule.
Experimental Set-Up
Two heteronuclear 2D experiments - HMBC and ME-HSQC - are demonstrated in this article. They are classified as inverse heteronuclear experiments, because the signals from hydrogen nuclei are directly observed, in order to increase sensitivity. This technique allows much faster data acquisition than with a direct heteronuclear experiment, such as HETCOR, often reducing the necessary time from multiple hours to just tens of minutes.
These experiments are demonstrated using a 1 M sample of the molecule gemfibrozil (see Figure 1). Gemfibrozil is a medication used for treating abnormal blood lipid levels.
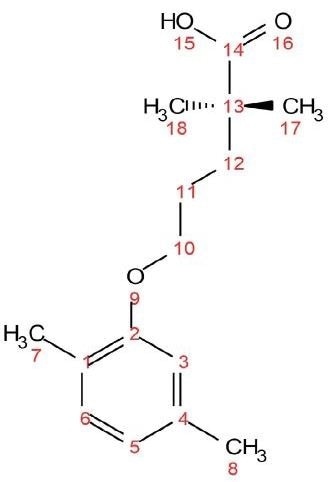
Figure 1. Chemical structure of gemfibrozil
Multiplicity Edited Heteronuclear Single Quantum Coherence (ME-HSQC) Experiment
- HSQC: In the HSQC, signals from nuclei of two different elements can be directly linked. In this case, the signal from a hydrogen nucleus can be associated with the signal from a carbon nucleus to which it is directly bound. This correlation manifests as crosspeaks (peaks appearing at frequencies on the hydrogen and carbon axes that correspond to peaks in the 1D hydrogen and carbon spectra) in the 2D HSQC spectrum. Signals in the 1D carbon spectrum without any crosspeak are instantly identified as quaternary carbons.
- Multiplicity-Edited HSQC: The multiplicity-edited, also called DEPT-edited, version of the HSQC is a well-known extension to the basic HSQC experiment. Similar to the HSQC experiment, the signal from the hydrogen nuclei and the signal from the carbon nuclei that are directly bound are joined via a crosspeak in the ME-HSQC spectrum. The multiplicity of the hydrocarbon group, that is, CH, CH2, and CH3, can also be directly established by the peak phase.
The CH and CH3 crosspeaks are positive (indicated as red in Figure 2), while the CH2 crosspeaks are negative (indicated as blue in Figure 2). The potential to rapidly detect the multiplicity is essential for structural elucidation. Figure 2 shows the full ME-HSQC spectrum for gemfibrozil.
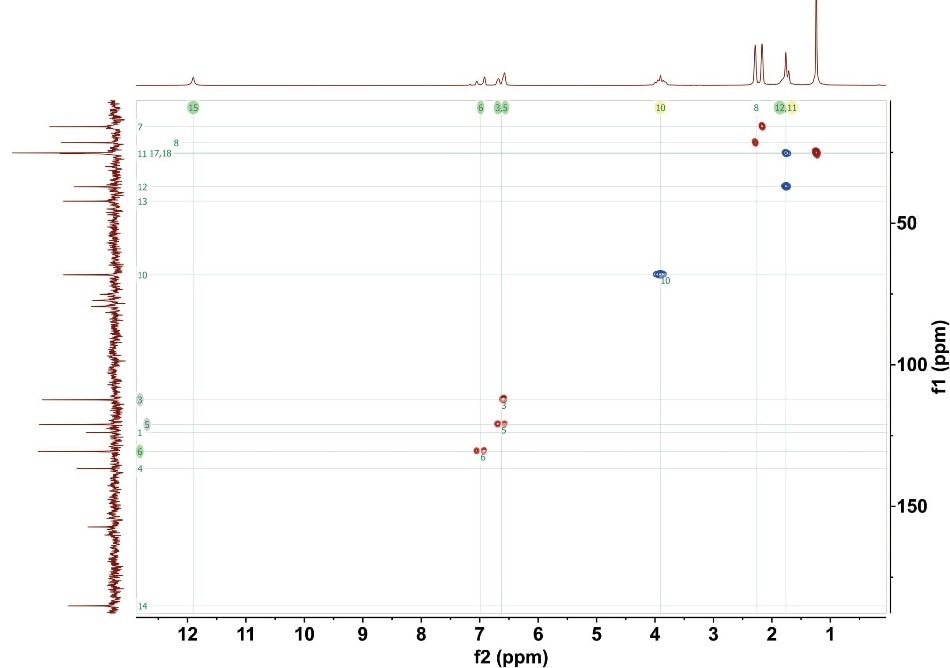
Figure 2. ME-HSQC for 1M gemfibrozil. 4 scans with total experimental time 35 minutes. The numbers on the crosspeaks correlate to the numbered carbon positions in Figure 1.
Heteronuclear Multiple Bond Correlation (HMBC) Experiments
- HMBC: The HSQC experiment offers useful data about the hydrocarbon functional groups, which is often sufficient for validating a known structure. But it is not sufficient for the structural elucidation of an unknown molecule. Generally, structural elucidation demands more data about the connectivity along the molecule’s carbon backbone. This data is provided by the HMBC experiment.
In the HMBC experiment, the signals from hydrogen nuclei are associated with the signals from carbon nuclei 2 and 3 bonds away. If part of a hydrocarbon chain 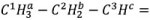 is considered, then the ME-HSQC would display crosspeaks between C1 and Ha, C2 and Hb, and C3 and Hc.
is considered, then the ME-HSQC would display crosspeaks between C1 and Ha, C2 and Hb, and C3 and Hc.
The HSQC crosspeaks in the HMBC spectrum will be suppressed, while crosspeaks of C1 with Hb and Hc, C2 with Ha and Hc, and C3 with Ha and Hb will be seen.
Through the combination of both spectra, the NMR user can construct a picture of the carbon backbone structure as well as the related hydrocarbon groups. Figure 3 shows the entire HMBC spectrum of gemfibrozil.
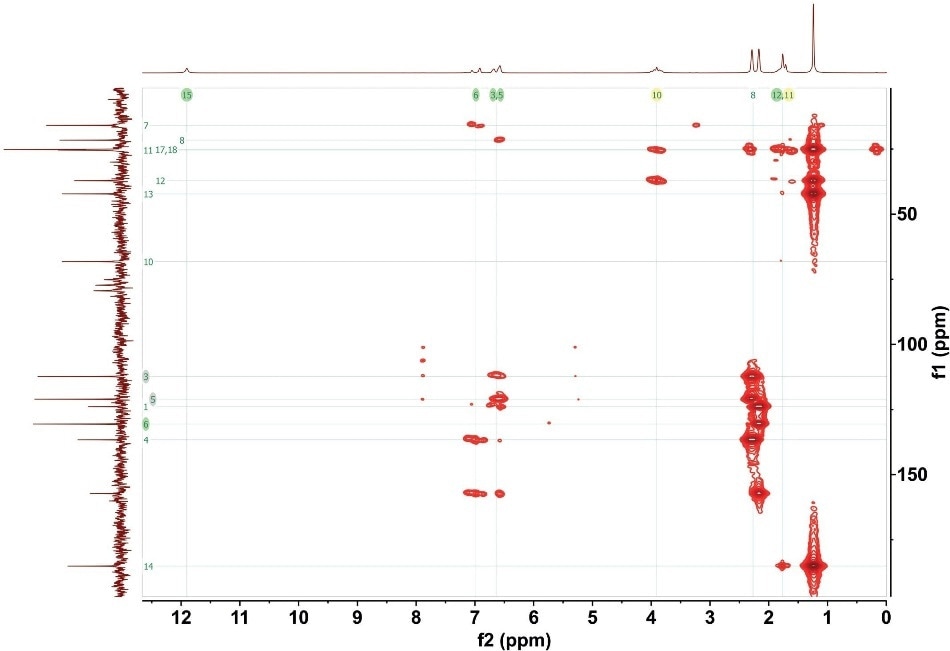
Figure 3. HMBC for 1M gemfibrozil. 16 scans with total experimental time of 1 hour 40 minutes.
Conclusion
Through heteronuclear correlation experiments, users can establish the structure of completely unidentified molecules by associating hydrogen atoms with the carbons they are bound to (ME-HSQC) and also with carbons 2 and 3 bonds away (HMBC).
The greater sensitivity of proton measurements can be used by inverse experiments like HMBC and HSQC to enable the structural elucidation of many unidentified compounds within a few hours.

This information has been sourced, reviewed and adapted from materials provided by Oxford Instruments Magnetic Resonance.
For more information on this source, please visit Oxford Instruments Magnetic Resonance.