
Dr. Tim Nunney, Product Marketing Manager at Thermo Fisher Scientific, Surface Analysis, talks about how surface engineering can help limit the spread of viruses and bacteria.
Unprecedented times call for unprecedented measures. With the emergence of COVID-19 at the start of the year, daily social rituals, such as a firm handshake and a tap on the shoulder have been stigmatised, and extra attention is drawn to importance of cleaning hands, not only after any physical interaction with another human being, but also after an interaction with surfaces. The screen of your phone, a door handle or a shopping trolley – all those objects can store and transmit viruses and bacteria from the area you touch onto your hands, and consequently increase the risk of developing an illness. The question is: what if we could alter surfaces to make them less capable of transmitting infections?
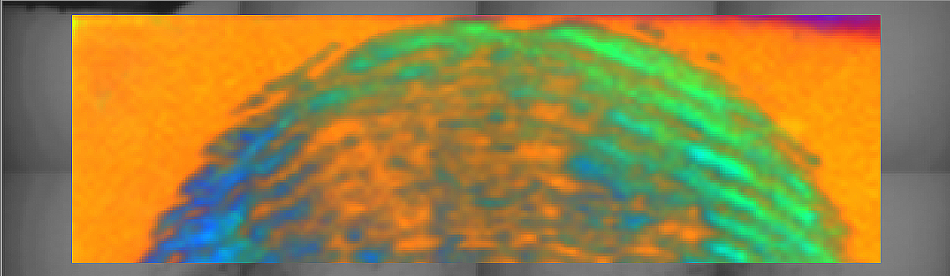
XPS image of a fingerprint on a silicon wafer. The green and blue colors indicate different chemical states present in the fingerprint, a result of what had been touched before the wafer.
First things first: when you touch a surface you leave behind fingerprints – which practically are a mixture of the oils from your skin, sweat, and any residue from anything you’ve touched earlier. By touching a contaminated object and then touching your eyes, nose, or mouth, there is the chance of catching COVID-19 (or any other virus type for that matter). The two variables here are how long the microbe remains active on that surface, and if another person touches that surface within that timeframe and get infected? COVID-19 seems to be quite adept at remaining active on certain materials for a long time. This is suggested to be one of the reasons why it seems to transmit so effectively.
How can we reduce this window of time where the virus remains active? This is when surface modifications come into play! What exactly can be done?
Method 1
Think about how easy it is to wipe fingerprints from the screen of your phone. The surface of the screen is typically treated with a coating so that fingerprints are easy to wipe off, usually fluoropolymer-based. This chemistry creates a superhydrophobic surface and can be used to ensure that virus particles and bacteria can’t adhere to a surface easily. This makes things easier to wipe clean, and disinfect, but probably won’t result in virus particles just sliding off.
Method 2
Treat the surface so that is can kill viruses and bacteria. This is the more commonly used approach, though there are several ways of doing it. For example, it’s quite common to see things such as wound dressings treated with silver, which has long-established anti-bacterial properties. Making things out of silver is prohibitively expensive but nano-particulate silver works too, which makes it cheaper. A less expensive solution is copper which also shows promising capabilities. It does, in fact, shorten the lifetime of COVID-19 to just a few hours as opposed to up to 48 hours for some materials. There are also technologies for introducing disinfecting capabilities to surfaces in the environment, either by releasing aerosol material which can deposit on surfaces and clean them up, or by spraying surfaces with micro-capsules.
Method 3
Adding a component that empowers a self-cleaning ability, such as titanium dioxide, which works as a photo-catalyst to break down organic material and uses light to promote the reaction. This results in the surface contamination being broken down and flaking off, hopefully deactivating the pathogen in the process.
The lifetime on a surface is still generally measured in hours, so don’t stop washing your hands just yet! But these developments in surface engineering continue to help with reducing the reproduction rate, and anything that reduces the chance of passing on bacteria is beneficial – especially in high-risk environments such as hospitals and care homes.
Do you have any experience in the area of self-cleaning or anti-bacterial surfaces? Share your experience in the comment section below!
Acknowledgments
I’m indebted to my colleague, Ksenia Tantsurina for her help in creating and editing this article.

This information has been sourced, reviewed and adapted from materials provided by Thermo Fisher Scientific – X-Ray Photoelectron Spectroscopy (XPS).
For more information on this source, please visit Thermo Fisher Scientific – X-Ray Photoelectron Spectroscopy (XPS).
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.