Most dry powder inhalers (DPI) operate with pre-metered doses of drug formulation held in a capsule or blister pack. The ability of dosator systems to deliver the required accuracy at high throughput means they are routinely selected to produce these packaged doses.
At the low volumes required, DPI formulations often exhibit properties that make them difficult to dose, with poor flowability a recognised issue. Reliable and efficient manufacture is dependent on choosing dosing technology that is well-matched to the properties of the formulation.
In this article Freeman Technology examines the properties of DPI formulations and the conditions they are subjected to during dosator operation. The article also reviews the benefits of multi-faceted powder characterization and how measuring powder properties can provide a basis for dosator selection for a given DPI formulation.
Dosing DPI formulations
The active pharmaceutical ingredient (API) must be in the region of five microns or less in size to reach the lung. Particles of this size are usually highly cohesive, which can make them difficult to process and disperse. A well-established strategy for addressing this issue, is to formulate the API with a relatively coarse carrier such as a lactose. The API is more easily dosed when attached to the relatively free-flowing carrier. During product use, the API is stripped from the carrier due to the force of the patient’s inhalation, drawing it into the lung and leaving the carrier in the mouth and/or throat.
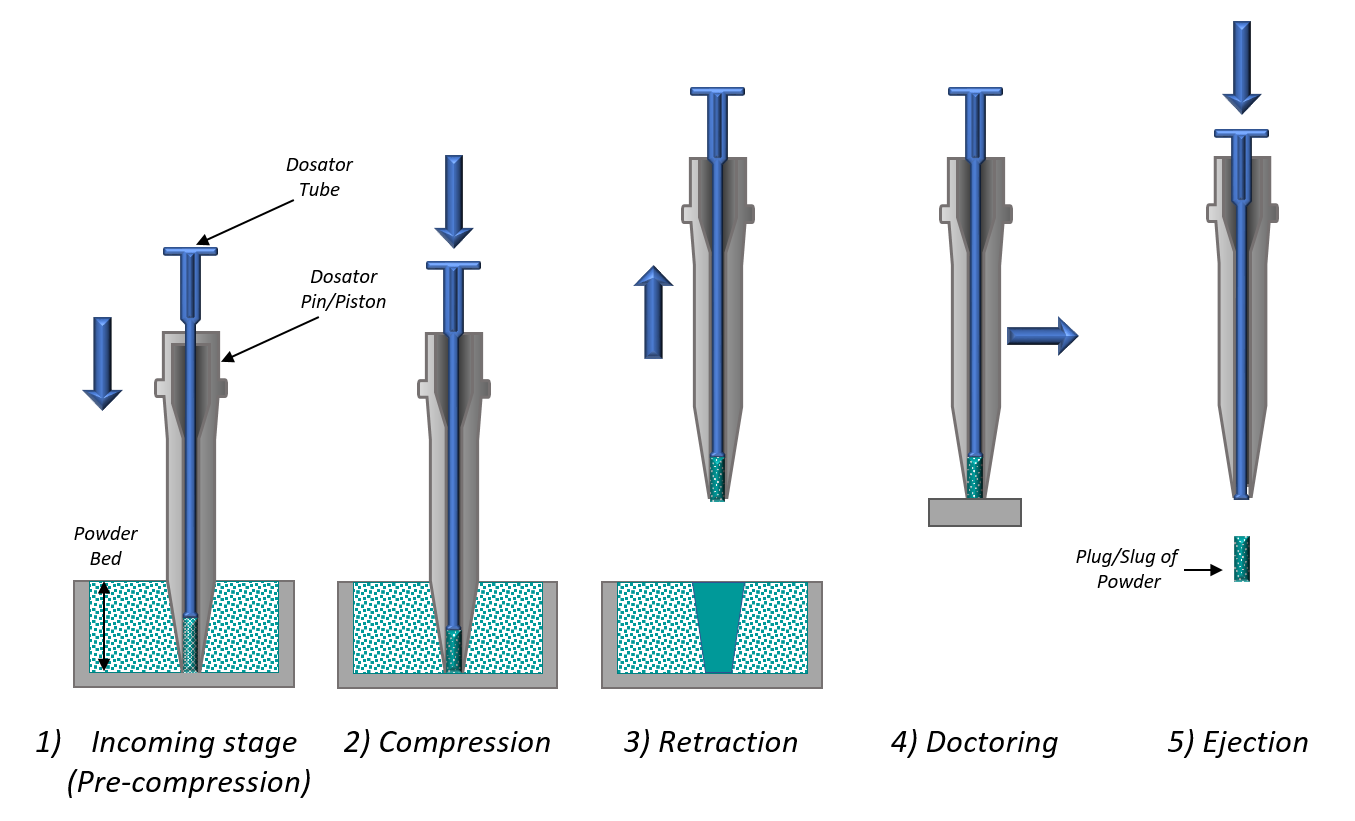
Figure 1: Dosator systems are routinely used to pre-meter DPI formulation doses.
Image Credit: Freeman Technology
Figure 1 shows a schematic of a dosator system. Powder flows into the open end of the dosator tube as it is pushed into the loosely packed powder bed. The dose is lightly compressed by the dosator pin, forming a compacted powder plug which is then ejected into the receiving capsule/blister. The dosing performance of a given dosator/powder combination, defined in terms of dose weight/uniformity, is influenced by the compressive force applied, the depth of the powder bed, and the initial height of the piston, as well as the ease with which the powder bed recovers following removal of a dose.
Using a powder rheometer for formulation characterization
Dynamic powder testing with an FT4 Powder Rheometer® assesses powders in motion and reports a flow energy to quantify resistance to flow. The technique enables differentiation of powders via process relevant data and can therefore assist in optimizing dosing technology.
Dynamic flow properties include:
- Basic Flowability Energy (BFE) – determined from precise measurements of the force and torque acting on a blade as it rotates downwards along a fixed path through a sample. BFE can be measured under different conditions in order to simulate various processes.
- Specific Energy (SE) – similar to BFE but measured during the upward traverse of the blade. SE is indicative of how the powder will behave when flowing in low stress conditions, for example, under gravity.
- Aerated Energy (AE) – also measured in a similar way to BFE but with air flowing through the sample. AE quantifies how aeration impacts flowability, up to the point of fluidization.
Case study: Using dynamic powder flow properties to optimize dosator performance

Figure 2: A lab scale dosator is useful for experimental investigations and confirmation of an optimal configuration.
Image Credit: Freeman Technology
Five lactose powders (particle size distributions shown in Table 1) were processed through a lab-scale dosator (Lab Dosator, 3P Innovation, Warwick, UK - Figure 2) using outlets of progressively decreasing size, from Dosator 1 to Dosator 4. All other process settings were kept constant. The target was to consistently produce doses of 50 mg with a relative standard deviation (RSD) of <2%.
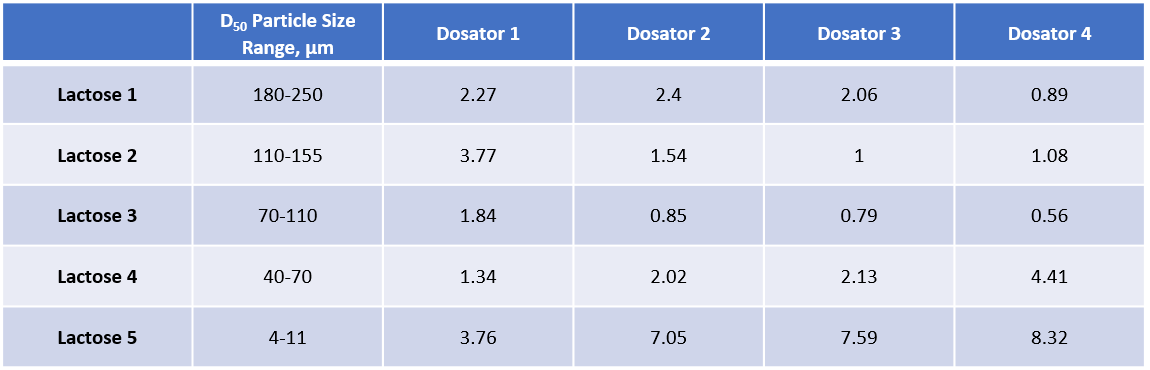
Table 1: Dosator performance for five lactose powders (expressed as %RSD).
Image Credit: Freeman Technology
Powder characterization
A range of dynamic, bulk and shear properties were measured for each of the lactose samples using the FT4 Powder Rheometer® (Freeman Technology). The measurements were used to rationalize the observed trends in dosator performance. AE and SE were found to correlate most strongly with dosator performance.
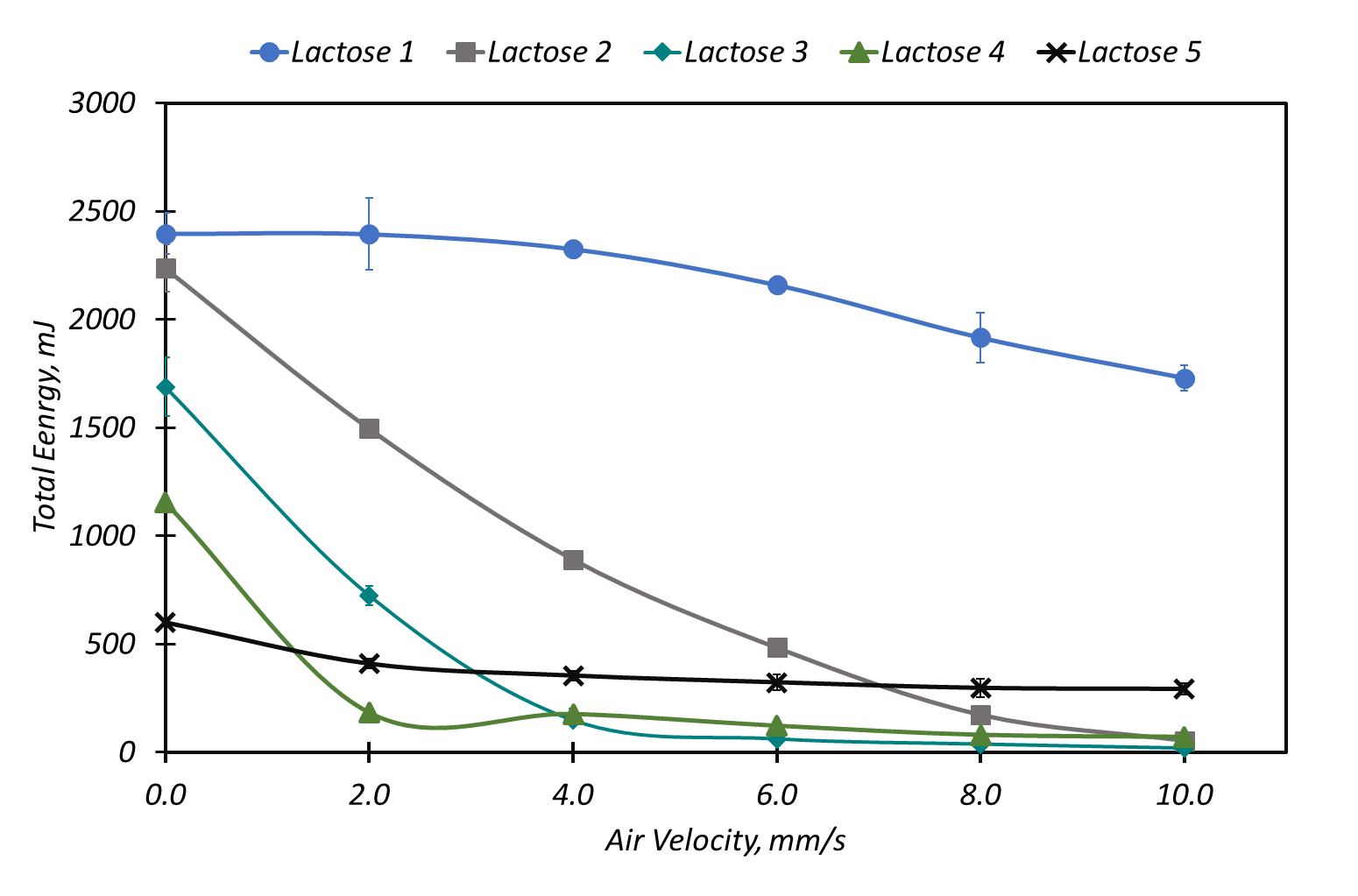
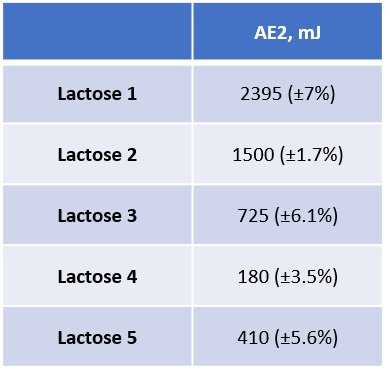
Figure 3: AE as function of air velocity, with AE at 2mm/s (AE2) data inset.
Image Credit: Freeman Technology
AE varies as a function of air velocity for all of the lactose samples (Figure 3); AE measured at 2mm/s highlights the different responses and was therefore evaluated with respect to dosator performance. The flow energies of Lactose 1 and Lactose 5 are substantially less impacted by air than those of the other samples. Samples 2, 3 and 4 all exhibit a similar profile but exhibit differences in AE2, which decreases from Lactose 2 to Lactose 4.
Fine, cohesive powders often generate lower flow energy values because of their ability to entrain air. Entrained air reduces the transmission of shear through the bed, thereby reducing the energy needed to move the powder (Figure 4). The subsequent introduction of air has little effect on these powders because the upward flowing air cannot easily overcome the strong inter-particular forces of attraction. This behaviour is exemplified by Lactose 5 which has a low flow energy and exhibits minimal change over the course of the test.
When inter-particular forces of attraction are lower, air flowing through the powder bed is able to separate individual particles. However, low inter-particular forces also reduce the ability to retain air. This behaviour is described by a high flow energy in a non-aerated state with a significant change following the introduction of air as exemplified by Lactose 2, 3 and 4.
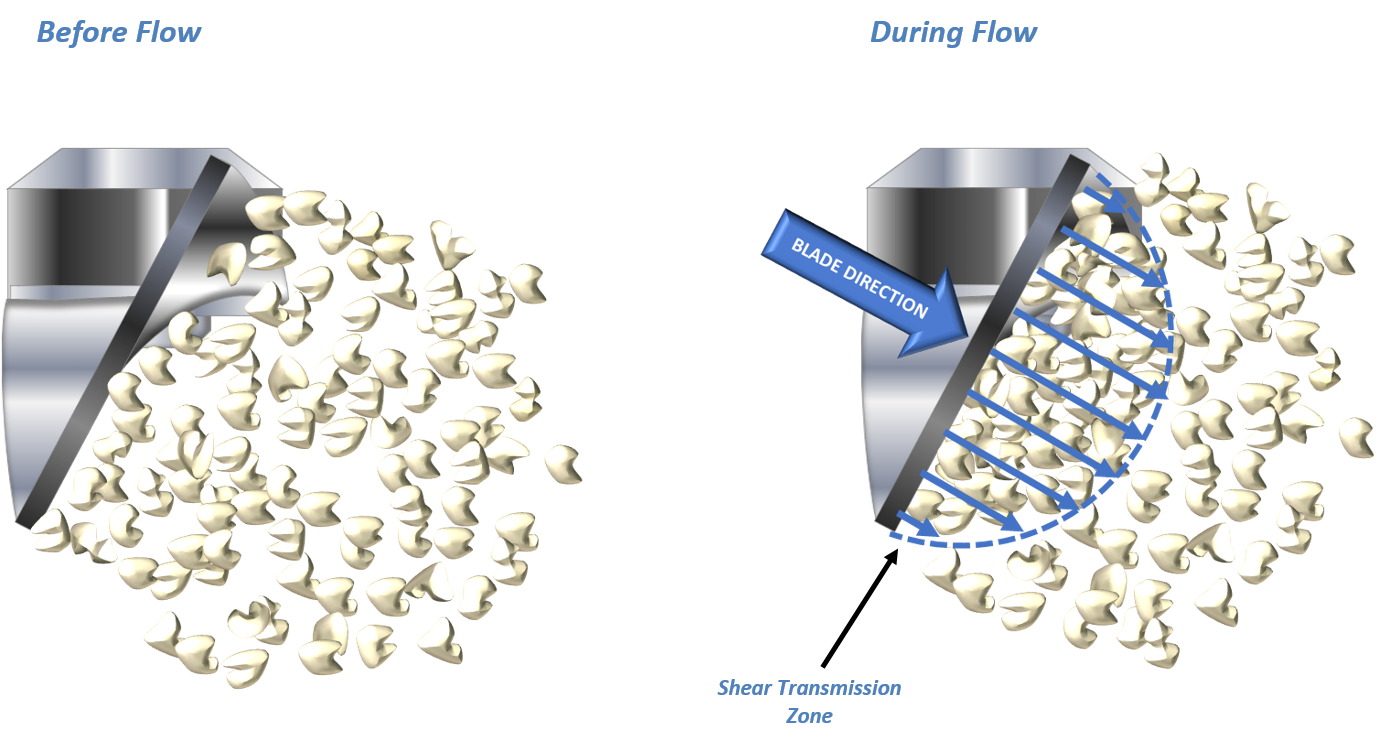
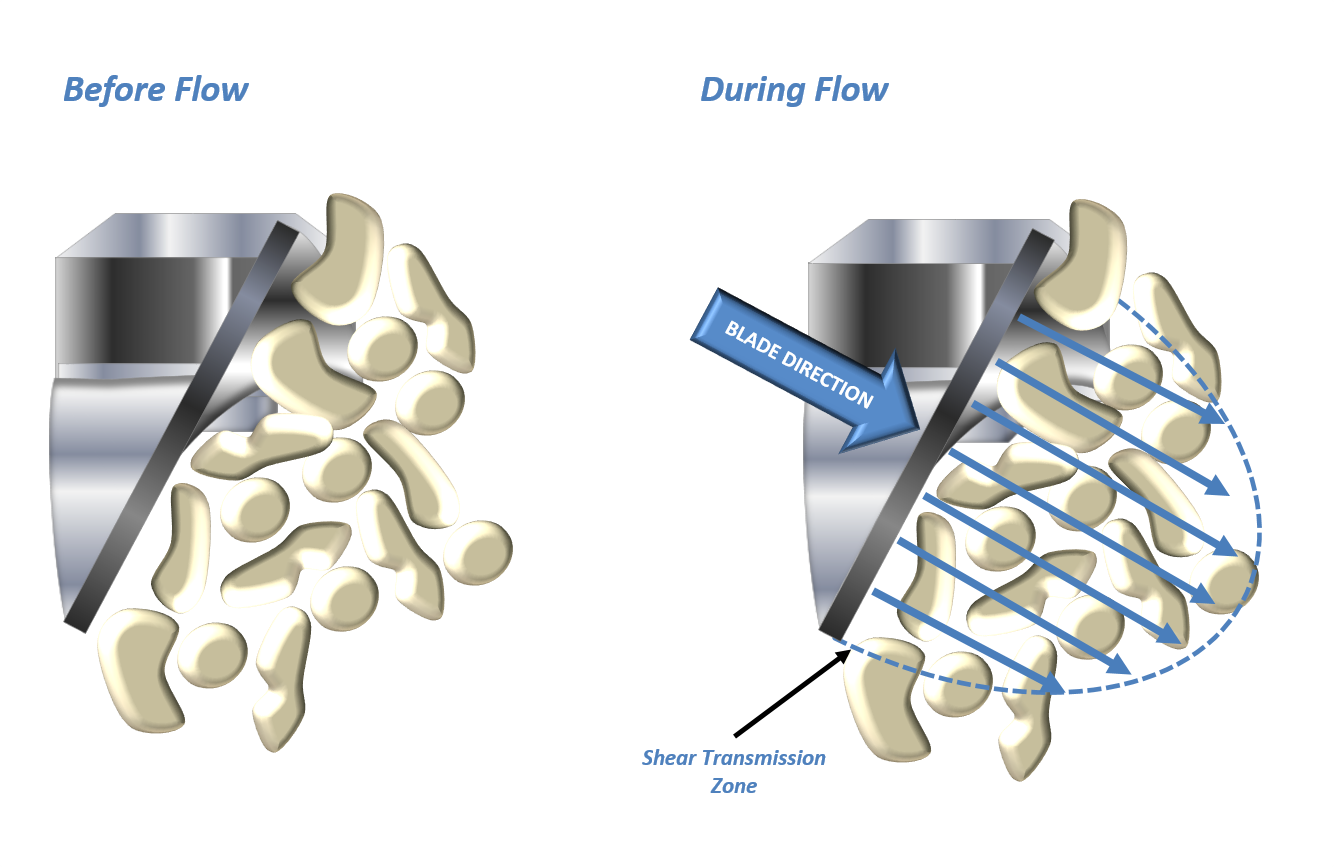
Figure 4: Air entrained in a cohesive powder dampens the shear transmission zone (top); lower inter-particular forces of attraction allow a more effective (bottom) transmission of shear.
Image Credit: Freeman Technology
A third pattern of behaviour can be observed in powders with sufficiently coarse and/orregular particles. The extremely efficient transmission of stress results in a high flow energy. However, a combination of low inter-particular forces and high permeability means that air flows freely through the bed with little to no impact on the packing structure. This characteristic is evident in Lactose 1.
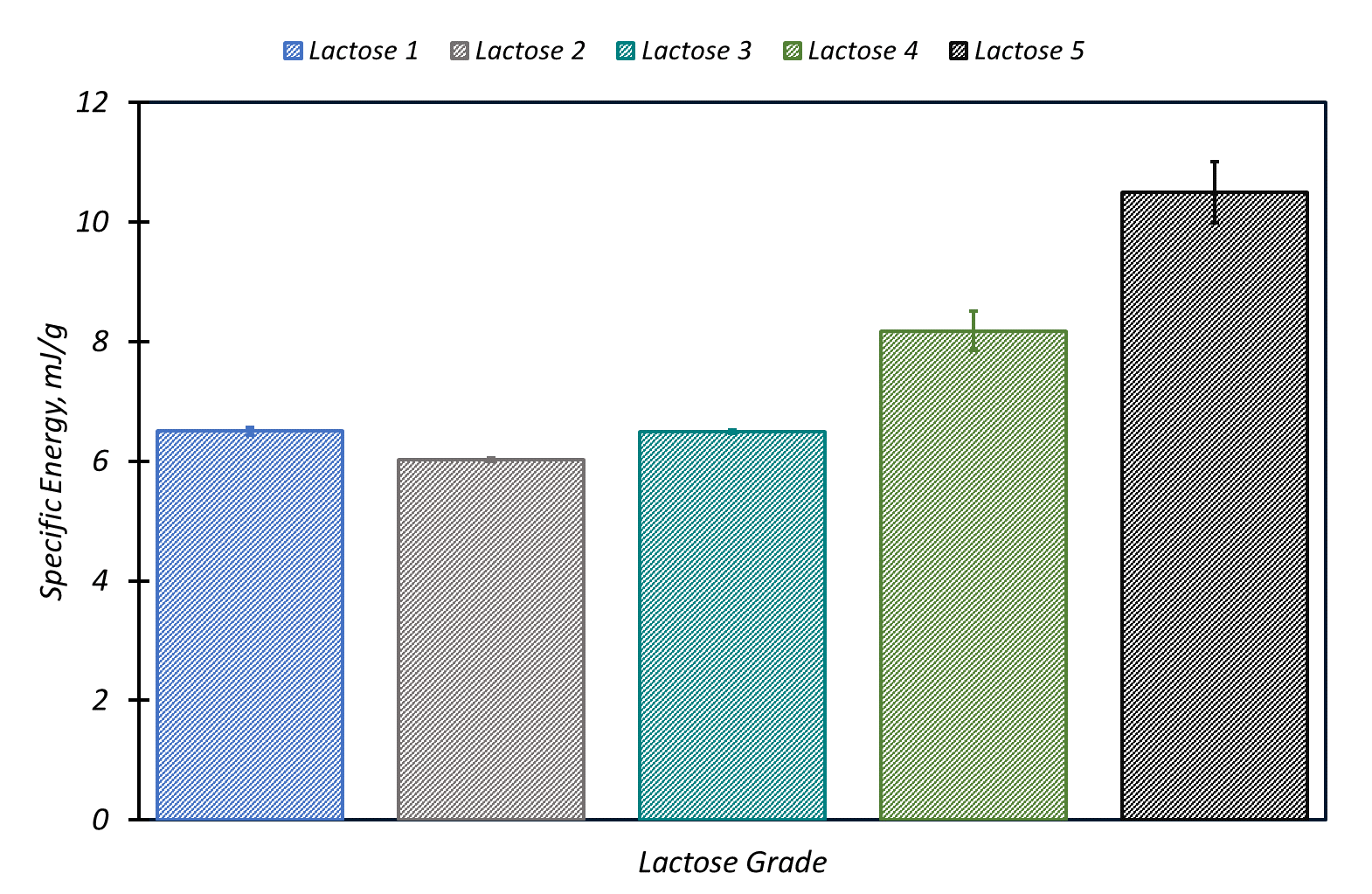
Figure 5: SE values indicate significant variation in inter-particular friction and mechanical interlocking.
Image Credit: Freeman Technology
Figure 5 shows SE values for the five lactose samples. SE measurements are highly influenced by inter-particular friction and mechanical interlocking as the powder is unconfined. Particles with an irregular or rough morphology may lock together, resulting in higher SE values. Lactose 5, the finest sample, exhibits the highest SE, while the two coarse samples, Lactose 1 and 2 have similarly low values.
Understanding dosator performance
Correlating dynamic properties with dosator performance enables specifications to be defined for powders that will process well in each dosator. Lactose 3 and Lactose 4 are the only samples to deliver acceptable performance in Dosator 1, which has the largest outlet. Both powders have relatively low AE2 values combined with a mid-range SE. Lactose 5 also has a low AE2, but its SE value is high. Dosator 1 is therefore suited to powders that combine a low AE2 with a moderately low SE.
Dosators 2 and 3 deliver acceptable performance with Lactose 2 and Lactose 3 and close to acceptable performance with Lactose 1 and Lactose 4. Lactose 2 and 3 are similar; low SE values and a similar AE profile. Lactose 1 has a similar SE but a much higher AE2 while the Lactose 4 has a low AE2 but a relatively high SE. Dosators 2 and 3 also require a powder with a relatively low SE 1 but can tolerate a higher AE2 than Dosator 1.
Lactose 1, 2 and 3 all exhibit acceptable performance in Dosator 4 but Lactose 4 and Lactose 5 perform poorly. Performance in this dosator is heavily influenced by SE with minimal correlation with AE2.
Powders with a combination of low SE with low AE2 perform better in all dosator configurations. However, as the outlet size of the dosator increases, AE2 becomes more influential and the impact of SE decreases. Dosators with larger outlets allow greater interaction with air, reducing the impact of inter-particular interactions. This is reflected in a stronger relationship with AE2. Smaller outlets provide less opportunity for interaction with air and the mechanical interactions between particles dictate performance.
In conclusion
DPI formulations are processed using different dosing techniques. Flow properties of a formulation will influence equipment choice and the resulting performance. This work demonstrates how dynamic flow testing with an FT4 Powder Rheometer delivers data that can be applied to better understand dosator performance and specify critical process parameters. This approach enables the development of formulations that are more easily processed and the selection of equipment that consistently deliver.
References:
1Podczeck, F and Jones, B ‘Pharmaceutical Capsules’ Second Edition, Published Feb 2004
2 Shur, J, Price, R and Freeman, T ‘Fine Tuning DPI Formulations’ Manufacturing Chemist, June 2008